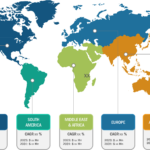
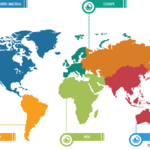
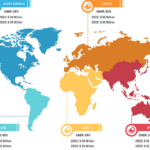
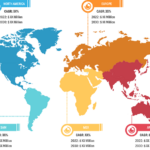
Product innovation plays a crucial role in shaping the market landscape of anesthesia monitoring devices, offering substantial growth, advancement, and enhanced patient care. The FDA officially authorized the PMD-200 monitor for sale in the US in March 2023. The device, developed by Medasense Biometrics, measures a patient’s nociception level (NOL), or physiological reaction to pain, […]
Headline
Tag: Anesthesia Monitoring Devices Market Trends
Back To Top