Biologics Market
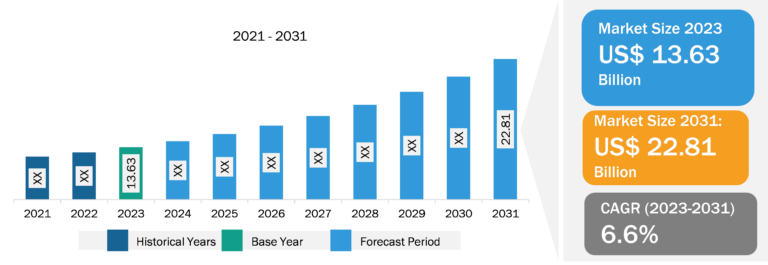
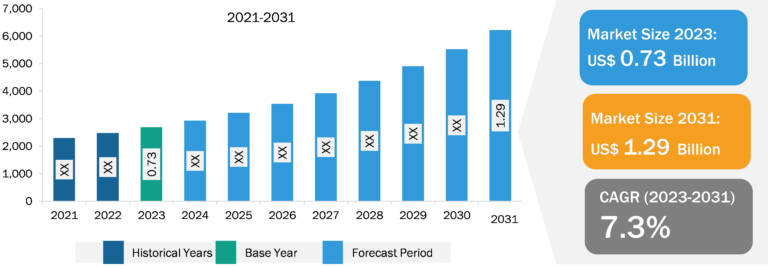
Biologics are among the most promising avenues in the pharmaceutical industry, offering a unique approach to treating various diseases and medical conditions. The biologics market is constantly growing because pharma and biotechnology companies enormously invest in improving their production facilities. The increasing number of cases and diagnoses of chronic diseases requires the availability of sophisticated diagnostic and treatment drugs, which has boosted the global biologics market. Furthermore, rising demand, higher acceptance of innovative therapies, and increasing clinical trials are expected to drive market growth. However, approving biosimilars is one of the main aspects limiting the biologics market expansion.
The market in North America is expected to register a CAGR of 18.4% during 2023–2031. This is due to the high prevalence of chronic diseases, numerous leading biopharmaceutical companies, favorable reimbursement policies, and significant investments in research and development. According to an article published in JAMA Network, biologics accounted for 37% of total drug spending in the US. The increasing share of prescriptions for biologics and growing investments in developing targeted drugs are contributing to the market growth. Additionally, approvals for several novel biological drugs such as antisense, gene therapy, and RNAi therapeutics are expected to drive market growth further.
The Asia Pacific biologics market is expected to record the fastest CAGR during 2023–2031. The increasing burden of diseases such as cancer, diabetes, and cardiovascular diseases in Asia Pacific, coupled with the growing geriatric population, has increased the demand for biologics. Industry leaders place great emphasis on meeting this demand and are investing significantly in the development of advanced biologics. A key driver of market growth in this region is the introduction of biosimilars, which play a critical role in improving the accessibility and affordability of biologic therapies.
Evolution of Biotechnology Industry in Developing Regions to Provide Market Opportunities
In modern biotechnology, antibodies are required in numerous processes, from basic research to innovative medical evaluations. The development of therapeutic antibodies has opened new avenues in treating various diseases, demonstrating the transformative ability of these particles. Antibodies are also crucial in antibody-based diagnostics as they provide accurate and reliable approaches to disease identification. Growing support from governments, rising public–private partnerships, continuously changing disease profiles, and increasing funding activities are widely enhancing the performance of the biotechnology industry in regions such as Asia Pacific, South & Central America, and the Middle East & Africa. Countries such as the UAE, Saudi Arabia, Oman, Muscat, Bahrain, Taiwan, Malaysia, India, China, Singapore, and Japan are among the most advantageous destinations for the biotechnology industry. Continuous advancements in the fields of biotechnology, genetics, and cell biology have contributed to identifying the potential of therapeutics for targeted therapies. As the biotechnology industry expands scientists’ toolboxes, the combination of basic research, translational studies, and clinical trials will continue to develop, investigate, and test additional compounds, techniques, and therapeutic pathways. Biotechnology is a strategic sector for China. The Made in China 2025 initiative aims to produce high-tech products, including innovative medicines. The plan introduced targets for Chinese pharmaceutical companies to spur biotechnology innovation and increase exports. Furthermore, these Asian countries are more focused on using genomics, proteomics, and biomarker advancements for diagnostic and therapeutic applications. Therefore, the ongoing evolution of the biotechnology industry in developing regions is expected to create opportunities for the biologics market in the coming years.
High Cost associated to Biologics to Restrain the Market Growth
Biologics, particularly mAbs, are among the highest-priced pharmaceutical products worldwide. In the US, the median price range for the mAb treatment ranges from approx. US$ 15,000–200,000 annually. With an annual treatment price of approximately US$ 100,000, the most expensive mAbs are for oncology and hematology. According to Biomatik, the average cost of producing monoclonal antibodies ranges from US$ 6,000 to US$ 15,000. High costs are a major obstacle for pharmaceutical and biopharma companies. In addition, there are additional challenges and costs associated with the validation and evaluation of the custom antibodies. If the validation shows negative results, repetitive procedures are performed, which incurs huge additional costs. Therefore, even at a substantial discount scheme, mAbs remain too expensive for most of the population groups.
Further, advocacy is essential in promoting supportive policy regarding mAbs for enhancing availability. For underpinning all efforts to make mAbs more widely available, a need to increase awareness among governments, ministries of health, and patient advocacy groups at an individual and public health level is essential. In addition, companies focusing on high-income country markets have little incentive to pursue lower-cost strategies for developing, manufacturing, and delivering mAbs. Also, competition, regulation, and other strategies that can lower mAb prices are not sufficient to make products affordable worldwide. Thus, the high cost of biologics is limiting its market growth.
Biologics Market: Drug Class Overview
Based on product, the biologics market is divided into monoclonal antibodies, vaccines, recombinant hormones/proteins, cell and gene therapy, and others. The monoclonal antibodies segment held the largest market share in 2023 and is likely to register the highest CAGR during 2023–2031.
By application, the market is segmented into cancer, infectious diseases, autoimmune diseases, and others. The cancer segment held the largest share of the market in 2023 and is anticipated to register the highest CAGR during 2023–2031.
In terms of manufacturing, the market is bifurcated into outsourced and in-house. The outsourced segment held a larger share of the market in 2023 and is estimated to register a higher CAGR during 2023–2031.
In terms of source, the biologics market is segmented into mammalian and microbial. The microbial segment held a larger market share in 2023. The mammalian is estimated to record a faster CAGR during 2023–2031. Mammalian expression systems are mainly used to develop recombinant proteins and vaccines based on viral vectors. The most frequently used mammalian cell lines are CHO and HEK. Products such as Perjeta (pertuzumab), Adcetris (brentuximab vedotin), Kadycla (trastuzumab emtansine), Shingrix (zoster vaccine), and Aimovig (erenumab) are among the products manufactured in mammalian expression systems.
Biologics Market: Competitive Landscape and Key Developments
AbbVie Inc.; Pfizer Inc.; Samsung Biologics; ADMA Biologics, Inc.; WuXi Biologics; Catalent, Inc; AGC Biologics; Crescendo Biologics Limited; Zumutor Biologics INC; Theriva Biologics; AstraZeneca; Amgen Inc.; Avecia Biologics; and Quality Assistance s.a. are among the key companies operating in the biologics market.