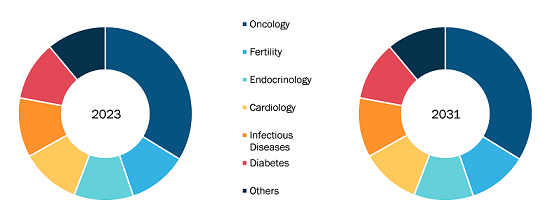
Prostate Cancer Therapeutics Market
The increasing number of prostate cancer cases and rising strategic initiatives by the prostate cancer therapeutics market players bolster the prostate cancer therapeutics market size. However, the high cost associated with prostate cancer therapeutics hinders the growth of prostate cancer therapeutics market growth.
Growing Demand for Targeted Therapy to Become Trend in Prostate Cancer Therapeutics Market During Forecast Period
Targeted therapy, a personalized medicine, combines two or more drugs designed to target the cancerous cells directly. Therapies such as ablation and chemotherapy destroy healthy and infected cells, affecting the overall patient condition. Targeted therapy is designed to selectively eliminate cancerous cells, keeping the function of healthy cells unhampered. With recent developments in medical technologies, the focus on the use of targeted therapy for the treatment of prostate cancer is increasing notably. Scientists are focusing on studying prostate cancer cell mutations responsible for driving the uncontrolled growth of cancerous cells. In April 2023, the Food and Drug Administration (FDA) granted Lantheus Holdings Inc. and POINT Biopharma Global Inc. track designation for 177Lu-PNT2002 for treating metastatic castration-resistant prostate cancer (mCRPC). PNT2002 is an innovative PSMA-targeted 177Lu-based radiopharmaceutical therapy that combines PSMA-targeted ligand, PSMA-I&T, and beta-emitting radioisotope no-carrier-added 177Lu for treating mCRPC. In March 2022, the US FDA approved Novartis’s Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for treating adult patients suffering from a type of advanced prostate cancer called prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC). Growing awareness about the benefits of targeted therapy among patients is expected to fuel the prostate cancer therapeutics market growth during the forecast period.
The current landscape for prostate cancer therapies is promising, with multiple drug approvals, a rich pipeline, and many ongoing clinical trials. Multiple prostate cancer therapies are in various stages of clinical development; pharmaceutical giants are working to expand their pipeline, which would be a major opportunity prevailing in the prostate cancer therapy market in the coming years. A few of the prostate cancer therapies that are in different phases of clinical studies are mentioned below:
| Sr. No. | Manufacturer | Product Name | Clinical Trial Phase |
| 1 | AstraZeneca | capivasertib | Clinical Phase II |
| capivasertib + abiraterone CAPItello-281 | Clinical Phase III | ||
| 2 | Pfizer | PF-07220060 + enzalutamide | Clinical Phase I |
| PF-06821497 + enzalutamide | Clinical Phase II | ||
| TALZENNA | Clinical Phase III | ||
| 3 | Lilly | Abemaciclib | Clinical Phase III |
Source: ClinicalTrials.gov
Strong product pipeline for the treatment of prostate cancer using chemotherapy, targeted therapy, and combinational therapy is expected to fuel the growth of the prostate cancer therapeutics market in the future.
North America held the largest share of the global prostate cancer therapeutics market in 2022 owing to the increasing technological advancements, rising number of prostate cancer cases, government support for a growing product pipeline, and major market players engaged in new and existing product developments. However, Asia Pacific is anticipated to register the highest CAGR from 2022 to 2030. The US held the largest share of North America’s prostate cancer therapeutics market in 2022. The prostate cancer therapeutics market growth in the US is mainly driven by the increasing incidence of prostate cancer cases, product launches, and government initiatives. In June 2023, AstraZeneca and MSD’s Lynparza (olaparib) in combination with prednisone or prednisolone and abiraterone was approved in the US for the treatment of adult patients affected by suspected deleterious BRCA-mutated (BRCAm) metastatic castration-resistant prostate cancer (mCRPC). In addition, in November 2023, the FDA approved enzalutamide, a product that Astellas Pharma US, Inc. manufactured. The product is used for the treatment of non-metastatic castration-sensitive prostate cancer (nmCSPC) with biochemical recurrence at high risk for metastasis (high-risk BCR). Other than skin cancer, prostate cancer is one of the most common cancers diagnosed in American men. According to the American Cancer Society Inc, in 2023, ~288,000 new prostate cancer cases and about 34,700 prostate cancer deaths will be registered in the US. As per the same source, ~ 1 in 8 men will be diagnosed with prostate cancer during their lifetime. Thus, the increasing prevalence of prostate cancer in the US and growing awareness about early detection and treatment of prostate cancer in the country fuel the prostate cancer therapeutics market growth in the country.
Prostate Cancer Therapeutics Market: Competitive Landscape and Key Developments
Astella Pharma Inc., Johnson & Johnson Services Inc., Eli Lilly and Company, Bayer AG, Sanofi, Merck KGaA, AstraZeneca, Novartis AG, AbbVie, and Bristol Myers Squibb are a few key companies operating in the prostate cancer therapeutics market. Market players adopt product innovation strategies to meet evolving customer demands, thereby maintaining their brand names in the prostate cancer therapeutics market.
A few of the recent developments in the global prostate cancer therapeutics market are mentioned below:
- In November 2023, Astellas Pharma Inc. acquired Propella Therapeutics Inc, also acquiring PRL-02 (abiraterone decanoate), a next-generation androgen biosynthesis inhibitor developed by Propella to treat prostate cancer.
- In February 2023, Fusion Pharmaceuticals Inc. acquired an investigational new drug application (“IND”) from RadioMedix, Inc. (“RadioMedix”). The drug is in a Phase 2 clinical trial (the “TATCIST” trial) evaluating 225Ac-PSMA I&T, a small molecule targeting prostate-specific membrane antigen (“PSMA”) expressed in prostate cancers. The alpha-emitting radiopharmaceutical being evaluated in the TATCIST trial is named FPI-2265.