Fluorine-18 Market
Factors such as the heightened prevalence of chronic diseases and increasing use of nuclear imaging techniques propel the fluorine-18 market growth. However, the short shelf-life of fluorine-18 impedes the growth of the market. Further, the use of radioactive tracers in cancer diagnosis is expected to bring new trends in the market in the coming years.
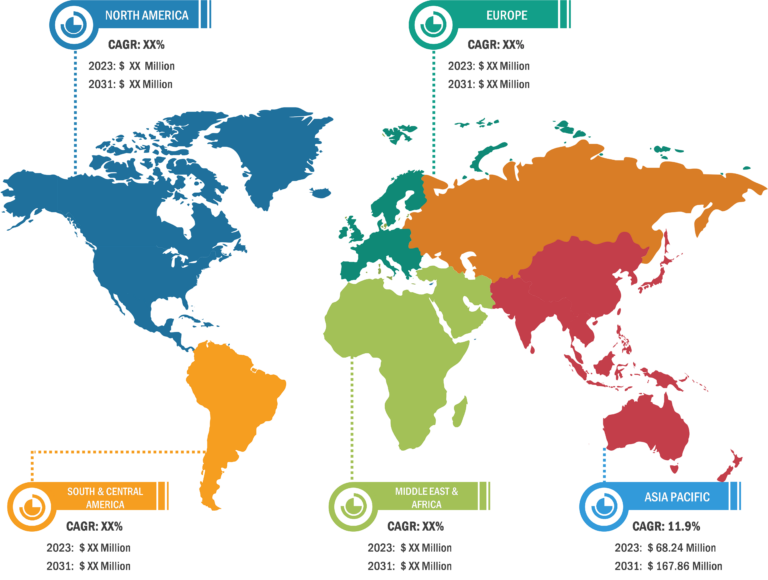
The fluorine-18 market in Asia Pacific is expected to grow at the highest CAGR during the forecast period. North America accounted for the largest share of the market in 2022. The US dominated the market in this region, in terms of share, in 2022, and it is estimated to continue its dominance during 2022–2030.
Increasing Initiatives for the Research and Development of Radioactive Tracers to Drive Fluorine-18 Market Growth in Future
Increasing initiatives for the research and development of radioactive tracers benefit the fluorine-18 market growth. The European Union Committee has taken various initiatives to boost R&D activities in the field of radioactive tracers and radioisotopes. The DOE Isotope Program (DOE IP) and its former organizations have been leading the development and production of radioactive and stable isotopes worldwide. Under the Atomic Energy Act of 1954, the DOE IP supplies isotopes and related services to the US, and it has the exclusive authority within DOE to produce isotopes for distribution and sales. The mission of the DOE IP is to conduct R&D to develop transformative isotope production, separation, and enrichment technologies to allow academic, federal, and industrial innovation, research, and emerging technologies. Further, many hospitals and research institutes focus on developing and using different radioactive tracers to diagnose chronic diseases. For example, Massachusetts General Hospital (MGH) is focused on using a new PET tracer [18F]3F4AP to diagnose multiple sclerosis, Alzheimer’s disease, mild cognitive impairment, and traumatic brain injury. According to the National Library of Medicine, [18F]3F4AP is also used in Phase 1 clinical trials to diagnose multiple sclerosis, Alzheimer’s disease, mild cognitive impairment, and traumatic brain injury. Thus, the ongoing research for discovering new radioactive tracers for diagnosing neurological disorders is further anticipated to fuel the fluorine-18 market growth in the coming years.
Short Shelf-Life of Fluorine-18 Hampers Growth of Fluorine-18 Market
The shelf-life of radiopharmaceuticals depends mainly on the half-life and radiochemical stability of radioisotopes, and the radionuclide impurity content in the preparation. Most radiopharmaceutical preparations contain radioisotopes with very short half-lives. For example, fluorine-18 must be used within ~110 min of preparation. The shelf-life of a multidose radiopharmaceutical preparation after aseptic withdrawal of the first dose depends on microbiological considerations. In case of a short half-life of radionuclides, the diagnostic radiopharmaceuticals are usually prepared in the hospital radiopharmacy departments just before the administration to patients. The half-life of fluorine-18 is 109.8 minutes, and the preparation contains only ~1% fraction after 12 hours, which is less than the half-life of other radiopharmaceuticals such as technetium-99m (6 hours). Therefore, short-lived radiopharmaceuticals are becoming a matter of concern in long-term procedures, which is a major factor restraining the fluorine-18 market growth.
Fluorine-18 Market: Segmental Overview
By product type, the market is segmented into FDG, NaF, and others. The FDG segment held the largest fluorine-18 market share in 2022 and it is anticipated to register the highest CAGR during the forecast period.
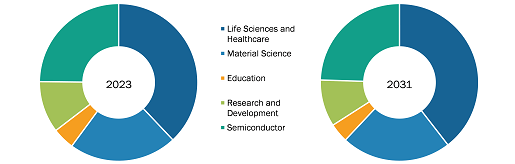
The fluorine-18 market, by application, is divided into oncology, cardiology, neurology, and others. The oncology segment held the largest market share in 2022, and it is anticipated to register the highest CAGR during the forecast period.
The market, by end user, is categorized into hospitals, diagnostic centers, and others. The hospitals segment held the largest fluorine-18 market share in 2022. It is anticipated to register the highest CAGR during the forecast period.
The fluorine-18 market report, based on geography, is segmented into North America (US, Canada, and Mexico), Europe (Germany, France, Italy, UK, Russia, and Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America).
Fluorine-18 Market: Competitive Landscape and Key Developments
Siemens Healthineers, Lantheus Medical Imaging Inc, Novartis AG, Cardinal Health, GE HealthCare, Jubilant Radiopharma, Eli Lilly and Company, Nukleon Nuclear Technology Research Ind Co Ltd, Curium Pharma, and Blue Earth Diagnostics Inc are among the prominent players profiled in the fluorine-18 market report. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the market.
A few of the recent developments in the global market are mentioned below:
- In October 2023, Blue Earth Diagnostics Ltd signed an exclusive strategic agreement with Sinotau Pharmaceutical Group for flotufolastat (18F) injection (formerly referred to as 18F-rhPSMA-7.3). Under the terms of the agreement, the companies became close strategic partners to join forces for technology and regulatory support for the distribution of flotufolastat (18F), a PET imaging agent, for Chinese men suffering from prostate cancer. This event marked an important phase in the Blue Earth Diagnostics’ strategy to make flotufolastat (18F) available to patients and clinicians globally.
- In July 2023, the European Commission granted marketing authorization for Pylclari, an innovative 18F-PSMA PET tracer from Curium, indicated for the detection of PSMA-positive (i.e., prostate-specific membrane antigen) lesions with PET in adults suffering from prostate cancer in the clinical settings.
- In May 2023, Blue Earth Diagnostics won the US Food and Drug Administration (FDA) approval for its optimized, high‐affinity radiohybrid (rh) PSMA‐targeted PET imaging agent—POSLUMA (flotufolastat F 18) injection (formerly referred to as 18F‐rhPSMA‐7.3). POSLUMA is indicated for the detection of PSMA-positive lesions via PET in prostate cancer patients, with suspected metastasis or suspected recurrence based on elevated serum prostate‐specific antigen (SA) level. Metastatic patients are considered as candidates for initial definitive therapy. POSLUMA was the first and only FDA‐approved, PSMA‐targeted imaging agent developed with proprietary rh technology. It was made commercially available in June 2023 through certain radiopharmacies in the national radiopharmacy network of Blue Earth Diagnostics, comprising its commercial manufacturers and distributors from the US.