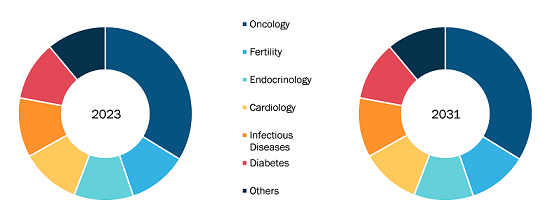
Monoclonal Antibody Therapeutics (mABs) Market
Production of mAB-Based Drugs to Treat Several Diseases Drives Market Growth
Monoclonal antibody therapeutics (mABs) are employed to treat a wide range of diseases, including cancer, autoimmune diseases, and metabolic diseases. Such drugs produced by biopharmaceutical companies and scientific research institutes have gained significant attention in the global market due to their high specificity, strong targeting ability, and low toxicity and side effects.
Therapeutic mABs Approved in European Union (EU) and US
| Products | Brand Name | Disease Indication | Approval Year: EU | Approval Year: US |
| Pozelimab | VEOPOZ | CHAPLE disease | NA | 2023 |
| Elranatamab | Elrexfio | Multiple myeloma | 2023 | 2023 |
| Rozanolixizumab | RYSTIGGO | Generalized myasthenia gravis | 2024 | 2023 |
| Talquetamab | TALVEY | Multiple myeloma | 2023 | 2023 |
| Epcoritamab | EPKINLY | Diffuse large B-cell lymphoma | 2023 | 2023 |
| Mirikizumab | Omvoh | Ulcerative colitis | 2023 | 2023 |
Source: Antibody Society
Monoclonal Antibody Therapeutics (mABs) Market Trend
Combination Drugs Containing Monoclonal Antibodies
According to the National Institute of Health (NIH) 2021 report, Roche and Regeneron (pharmaceutical companies) initiated the clinical trial phase 2/3 to evaluate combinational monoclonal antibodies for patients suffering from mild to moderate COVID-19. They are investigating “REGN-COV2,” a cocktail drug produced by combining two monoclonal antibodies—casirivimab and imdevimab—for the treatment of COVID-19. These companies expect that the combination of this mAB drug would reduce hospitalization by 70%, and it would be more effective on children above 12 years (having a body weight of more than 40 kg). Researchers are strongly looking for more such therapeutic combinations of monoclonal antibodies. For example, bamlanivimab and etesivimab developed by Elli Lilly have shown positive clinical results for COVID-19 in 2022. Therefore, combination drugs of monoclonal antibodies to treat several diseases would gain significant attention in the coming years, thus emerging as a prominent trend in the monoclonal antibody therapeutics (mABs) market.
Market Opportunity
Innovative Product Launches Through Strategic Developments by Manufacturers
Organic developments such as product launches by the manufacturers of therapeutic mABs are likely to bolster the monoclonal antibody therapeutics (mABs) market in the coming years. In March 2022, Adagio Therapeutics, Inc. announced the launch of ADG20 (ADINTREVIMAB). The newly launched product is the first monoclonal antibody to meet primary endpoints with statistical significance across pre- and post-exposure prophylaxis and treatment for COVID-19 by seeking US Emergency Use Authorization (EUA).
Further, inorganic developments such as mergers and acquisitions would result in the introduction of new therapeutic mABs. For instance, in July 2023, Elli Lilly announced the acquisition of Versanis, a private clinical-stage biopharmaceutical company intended to treat cardiometabolic diseases. Elli Lilly acquired Versanis to access its core product portfolio, including a monoclonal antibody product named bimagrumab. This product is currently being assessed in the “BELIEVE Phase 2b study” as a standalone molecule. It is also being studied in combination with semaglutide for its combined potential to reduce fat mass, preserve muscle mass, and deliver better patient outcomes in people living with obesity and obesity-related complications. The aforementioned factors are responsible for influential monoclonal antibody therapeutics (mABs) market growth in the coming years.
Monoclonal Antibody Therapeutics (mABs) Market: Geographic Overview
Based on geography, the monoclonal antibody therapeutics (mABs) market report is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2022, North America accounted for the largest monoclonal antibody therapeutics (mABs) market share. Asia Pacific is expected to register the highest CAGR during 2022–2030. In terms of revenue China dominated the monoclonal antibody therapeutics (mABs) market share. According to a report by the National Institute of Health (NIH), there is a rise in the approval of mAB therapeutics. This is due to infrastructure development in China, supporting mAB drug development studies and rapid product approvals by biopharmaceutical companies in China. Also, from August 2018 to December 2018, 4 PD-1 mAB drugs were marketed in China, 2 of which were domestically produced while the rest 2 were imported. Further, an NIH report reveals that the implementation of the Clinical Trial Management Policy has positively driven reforms in the clinical trial management system and support measures in China. Approximately, 70% of mAB drugs are indicated to treat cancer, where 4 (four) guidelines in 2012 were released supporting clinical trials of mAB drugs intended for cancer, and further the methodological guidelines for clinical trial design, execution, and data evaluation. Therefore, a well-developed infrastructure and supportive policies to develop mABs therapeutics are likely to accelerate the monoclonal antibody therapeutics (mABs) market in China and other Asia Pacific countries in the future.
Monoclonal Antibody Therapeutics (mABs) Industry Developments and Future Opportunities:
Various strategic developments by leading players operating in the monoclonal antibody therapeutics (mABs) market are listed below:
- In January 2023, AstraZeneca received approval for Evusheld in the European Union (EU). Evusheld is a combination of two long-acting antibodies—tixagevimab (AZD8895) and cilgavimab (AZD1061). The US government extended support for the development of this product through federal funds from the Department of Health and Human Services, the Administration for Strategic Preparedness and Response, and the Biomedical Advanced Research and Development Authority.
- In June 2020, Novartis received US FDA approval for ofatumumab intended for patients suffering from multiple sclerosis. Developed by Genmab and licensed by GlaxoSmithKline, Ofatumumab (OMB 157) is an anti-CD20 mAB sourced from human cell lines.
- In August 2023, Regeneron Pharmaceuticals, Inc. entered into an agreement with the Biomedical Advanced Research and Development Authority (BARDA) to support clinical development, clinical manufacturing, and the regulatory licensure process for next-generation monoclonal antibody therapy against COVID-19. Under this agreement, Regeneron plans to work collaboratively with BARDA to evaluate and further develop and manufacture this therapy, and conduct regulatory activities.
Monoclonal Antibody Therapeutics (mABs) Market: Competitive Landscape and Key Developments
The monoclonal antibody therapeutics (mABs) market analysis is carried out by identifying and evaluating key players in the market across different regions. GlaxoSmithKline, F.Hoffmann-La-Roche, Bayer AG, Amgen, Novartis, AbbVie, Bristol-Myers Squibb, Janssen Pharmaceutical, Merck KgaA, and AstraZeneca are the prominent players profiled in the monoclonal antibody therapeutics (mABs) market report. These companies focus on introducing new technologies, upgrading existing products, and undertaking geographic expansions to be able to address the globally increasing consumer demand.