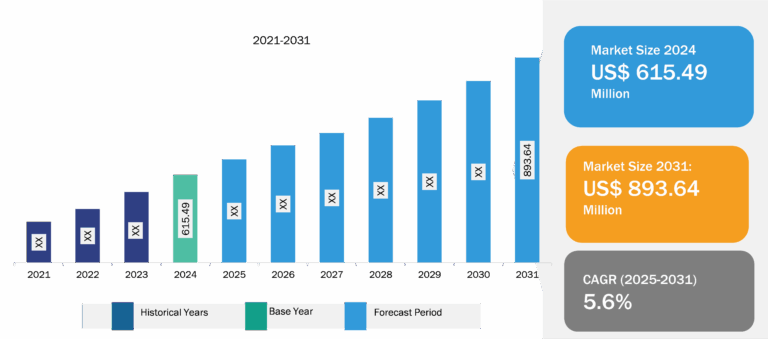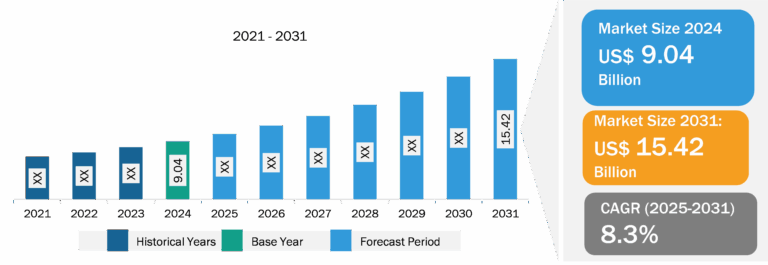
CRISPR and Cas Gene Market
CRISPR and CRISPR-associated system (Cas) technologies allow geneticists and medical researchers to alter parts of a genome by adding, removing, or changing sections of the DNA sequence. This type of genetic modification has emerged as the most versatile, simplest, and precise method. CRISPR and Cas are tools that have the potential to treat a range of diseases at genetic levels; these include cancer, hepatitis B, and high cholesterol. Many of the proposed applications involve editing somatic (non-reproductive) cell genomes. Nevertheless, there has been great interest and debate about the possibility of editing germline (reproductive) cells. Factors such as the increased investments by biotechnology companies in new treatments; continuous increase in genomic research and development; and an upsurge in the burden of cancer, hepatitis B, and so on are facilitating the expansion of the CRISPR and Cas gene market. However, the use of CRISPR in drug development and therapeutic procedures has been limited by its possibility of going off target, which can lead to unwanted changes to the genetic material. Further, increasing research funding for genomic studies and efforts made to demonstrate the effectiveness of CRISPR in genomics are expected to bring new trends in the CRISPR and Cas gene market in the coming years.
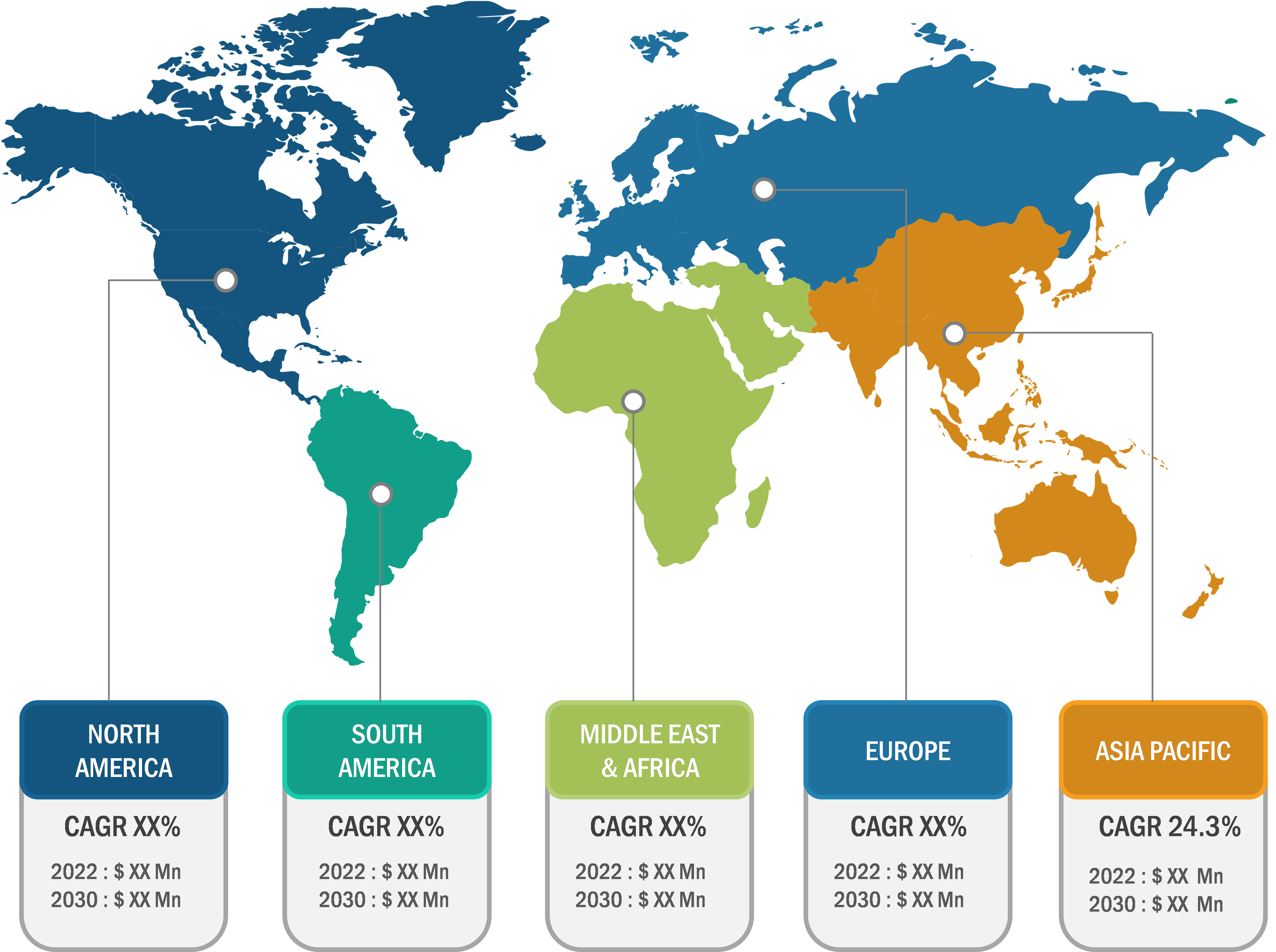
North America accounted for a major share of the global CRISPR and Cas gene market in 2022. The North American market is segmented into the US, Canada, and Mexico. The region holds a large share of the global market due to the growing biopharmaceutical research and development and the involvement of several pharmaceutical companies in the development of novel therapeutics. In May 2020, Merck KGaA received approval from the US Food and Drug Administration (FDA) for two CRISPR Cas9-driven gene editing patents. In addition, various federal initiatives in the US and Canada support agricultural biotechnology research and the commercial release of CRISPR-based plant products. Therefore, growth strategies such as partnerships, acquisitions, and collaborations to advance pharmaceutical developments and crop growth are expected to accelerate the CRISPR and Cas gene market progress during the forecast period.
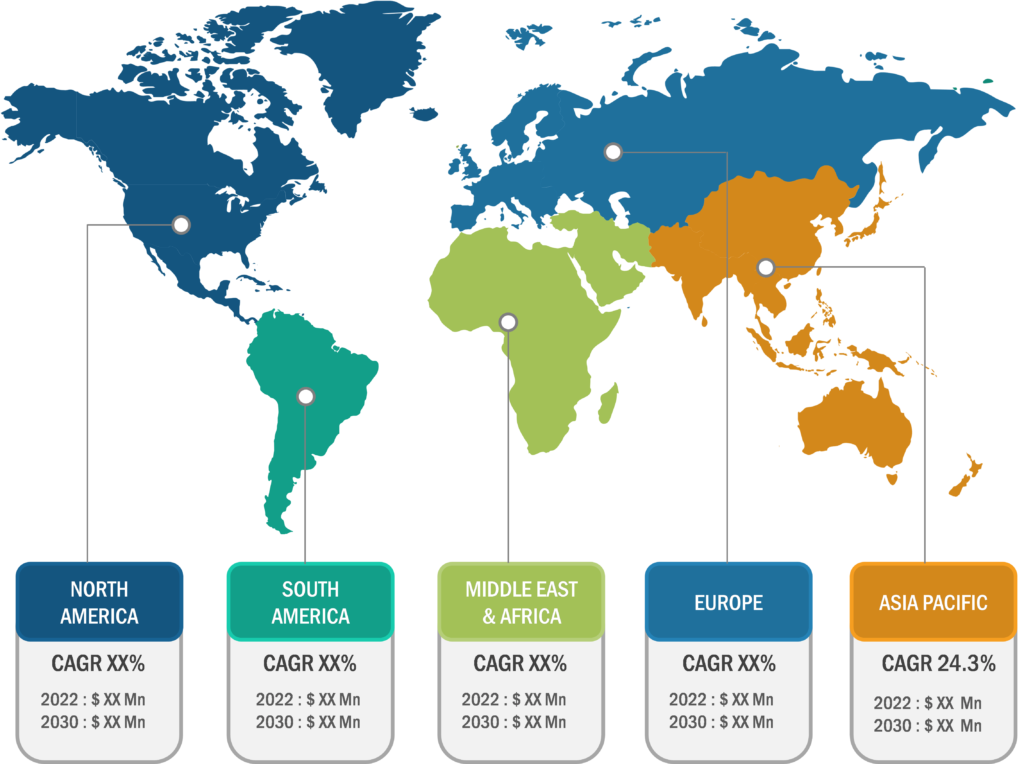
Asia Pacific is expected to register the fastest CAGR in the global CRISPR and Cas gene market during 2022–2030. The market growth in this region is ascribed to increasing government investments in research and development to develop novel treatments. Companies and research institutes in China are conducting research on genome editing for drug development, and they have recently announced several clinical trials to seek approval for the use of CRISPR in cancer treatment. In March 2021, Setsuro Tech, a Japanese biotech company, received a patent for CRISPR-Cas9 technology developed by Dublin-based ERS Genomics. Such initiatives are expected to propel the adoption of CRISPR technology in Asia Pacific in the future.
Off-Target Effects and Ethical Concerns Associated with CRISPR Technology Limit Market Progress
Although the CRISPR/Cas system is highly precise, it is imperfect, and it is associated with the risk of off-target effects. The relatively common off-target effects (OTEs), occurring at a frequency of 50%, pose a serious problem while using CRISPR/Cas9 in gene therapy. This has raised concerns regarding the safety and ethical implications of this technology and its use in gene editing. Off-target effects can lead to accidental and potentially harmful changes to the genome.
In addition, there are concerns about the possible abuse of the CRISPR and Cas gene technology for non-medical purposes, such as improving human characteristics. There are increasing ethical and safety concerns surrounding CRISPR and Cas gene therapy, gene editing, and genetically modified foods or organisms. Altering the human germline is the most important ethical issue related to CRISPR technology. CRISPR technology aims to correct a faulty human gene to treat certain diseases. Several organizations have expressed concerns that the use of CRISPR technology could alter humans’ natural DNA, which is unethical. These concerns are expected to lead to an increase in regulatory scrutiny and slower technology adoption, thereby hampering the CRISPR and Cas gene market growth.
CRISPR and Cas Gene Market: Segmental Overview
Based on product & service, the CRISPR and Cas gene market is divided into products and services. The products segment held a larger market share in 2022. It is anticipated to register a higher CAGR of 23.9% during the forecast period.
Numerous cutting-edge technologies such as CRISPR and Cas gene editing kits have been developed to meet the growing demand for genome editing solutions. An upsurge in the share of these kits in the overall market is attributed to the availability of improved single products that can achieve different goals, including simple gene knockouts, reduced off-target cutting, selective genome cleavage, genome engineering, and higher specificity.
By application, the CRISPR and Cas gene market is bifurcated into biomedical and agriculture. The biomedical segment held a larger market share in 2022. The same segment is anticipated to register a higher CAGR of 23.8% during the forecast period.
The CRISPR and Cas gene market, by end user, is categorized into academics and government research institutes, biotechnology and pharmaceutical companies, and contract research organizations (CRO) and CDMOs. The biotechnology and pharmaceutical companies segment held the largest market share in 2022. The same is anticipated to register the highest CAGR of 24.2% during the forecast period.
CRISPR and Cas Gene Market: Competitive Landscape and Key Developments
CRISPR Therapeutics; Thermo Fisher Scientific; Merck; GenScript; Qiagen; Takara Bio Inc.; Brain Biotech AG; Intellia Therapeutics, Inc.; New England Biolabs; and Editas Medicine, Inc. are among the key companies operating in the CRISPR and Cas gene market. Leading players are implementing strategies such as expansions, new product launches, and acquisitions (companies or new clientele) to tap prevailing business opportunities.
- In June 2021, QIAGEN—a provider of sample and testing technologies for molecular diagnostics, applied testing, and academic and pharmaceutical research—announced the launch of the QIAprep& CRISPR kit and CRISPR Q-Primer Solutions, which will help researchers analyze edited genetic material.
- In March 2021, Integrated DNA Technologies (IDT), a comprehensive genomics solutions provider, launched the rhAmpSeq CRISPR analysis system, an end-to-end solution to characterize and quantify the full spectrum of on- and off-target genome editing outcomes.
- In December 2020, Intellia Therapeutics, Inc. released preclinical data for NTLA-5001, the company’s wholly owned Wilms tumor 1-targeted T-cell therapy candidate for treating acute myeloid leukemia.
- In August 2020, Editas Medicine reclaimed the full ownership of a series of CRISPR-based gene editing investigational drugs from AbbVie Inc. for the treatment of eye diseases.
- In May 2020, Sherlock Biosciences received the FDA’s Emergency Use Authorization for its Sherlock CRISPR SARS-CoV-2 kit, designed to identify the virus causing COVID-19.