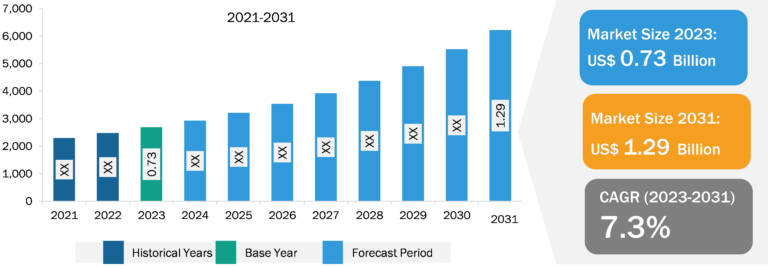
Bio-implants Market
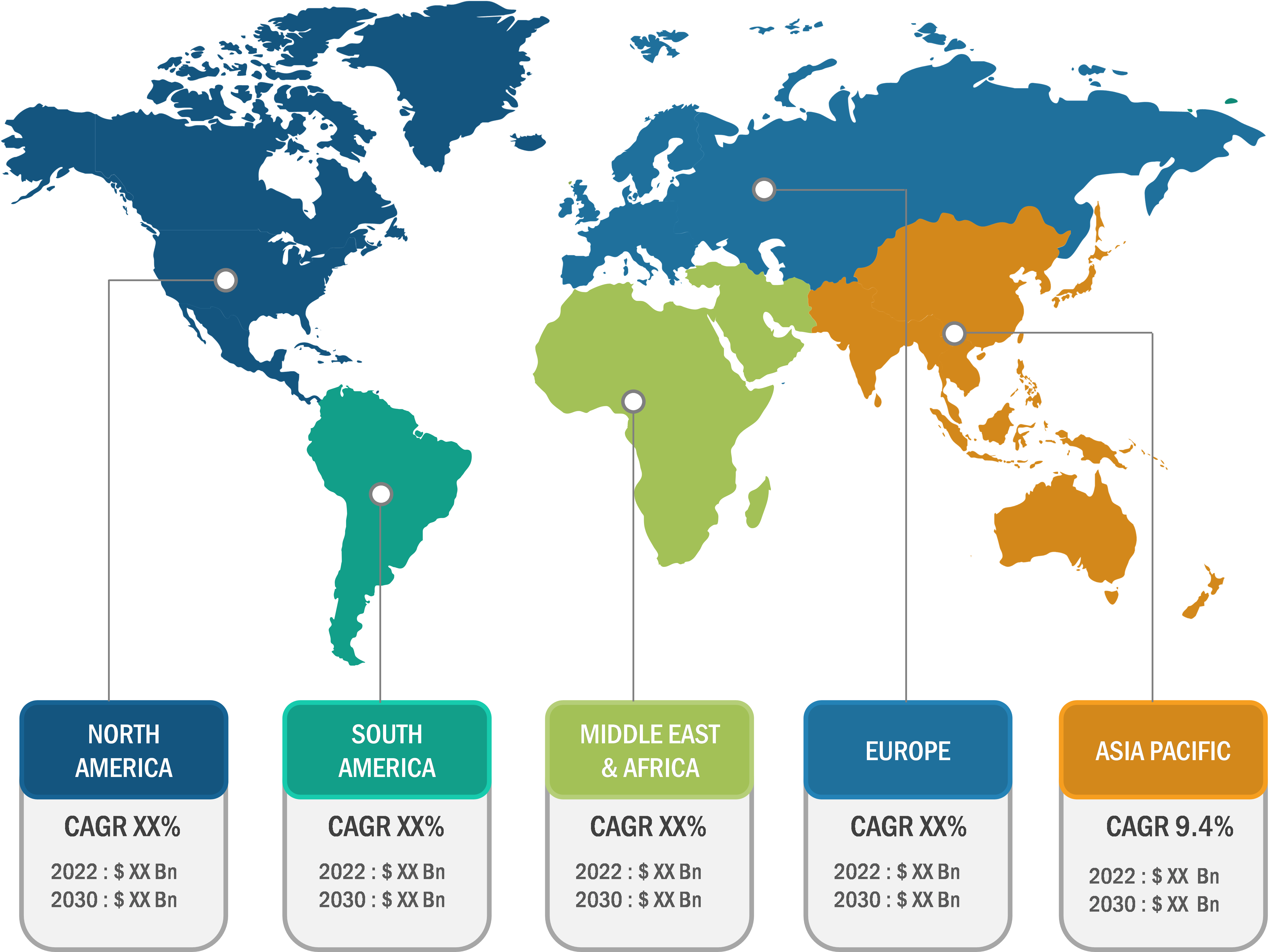
North America accounted for the largest market share of the global bio-implants market in 2023. The market in this region is segmented into the US, Canada, and Mexico. The growth of the bio-implants market in the region is due to the growing prevalence of chronic diseases and better healthcare infrastructure. In July 2022, updated Centers for Disease Control and Prevention (CDC) data shows that coronary artery disease is one of the most common types of heart diseases, with approximately 20.1 million adults aged 20 and older living with the disease in the US. Additionally, according to CDC data, every 40 seconds, an individual suffers from a heart attack in the US, i.e., nearly 805,000 people. The rising incidence of chronic diseases is expected to increase the overall demand for bio-implants, which is expected to boost the market growth during the forecast period.
Asia Pacific is expected to register the highest CAGR in the bio-implants market during 2023–2031. The market growth in the region is ascribed to the growing geriatric population, increasing disposable income, rising healthcare investments and expansion by market players, and increasing cases of spinal cord injuries due to the rising number of traffic accidents. Asia Pacific is experiencing significant growth, particularly in emerging markets such as China and India. Expanding healthcare infrastructure and increased investments in this region in order to provide efficient patient services fuel the market growth in Asia Pacific. Therefore, the proliferating healthcare industry, especially in Japan, South Korea, India, and China, is anticipated to catalyze the growth of the bio-implants market in Asia Pacific during the forecast period.
Increasing Technological Developments and Government Initiatives to Provide Market Opportunities in Coming Years
Technological developments such as 3D printing, laser technology, and nanotechnology have significantly improved the production of bio-implants. 3D printing has transformed the designing and manufacturing methodologies of bio-implants. This technology enables the creation of patient-specific implants with intricate geometries, precise dimensions, and tailored features, resulting in better fit and functionality. Further, integrating sensors, microelectronics, and wireless communication into implants helps in the real-time monitoring of the patient’s condition and health. Smart implants can transmit data to healthcare providers, enabling remote monitoring and timely interventions.
Many government organizations work with companies conducting health studies and producing medical devices to market new and more effective devices. For example, in December 2023, Nanyang Technological University Singapore and Singapore General Hospital (SGH) collaborated to invest in the advancement of 3D printing. The partnership leverages the facilities and combined expertise of the Singapore Center for 3D Printing at NTU and the 3D Printing Center at SGH to research and develop relevant technologies for clinical applications in point-of-care settings. In February 2024, a Pittsburgh engineer received US$ 557,000 from the National Institutes of Health to conduct the world’s first in vivo studies of orthopedic metamaterial implants to improve spinal injuries’ treatment, repair, and recovery. Metamaterials are more advanced than traditional elements, alloys, or other materials because they can be designed to provide a wide range of desired mechanical properties, including ultralight, ultra-stiff, ultrahigh strength-to-density ratios, compliance, and high resilience. In addition, metamaterial implants offer great scope for design as they can be made from various biocompatible materials. Thus, increasing technological developments and government initiatives are anticipated to provide growth opportunities for the market growth during the forecast period.
Bio-implants Market: Type Overview
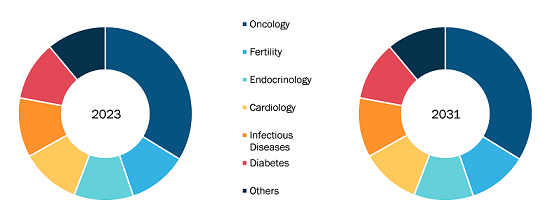
Based on product, the bio-implants market is segmented into cardiovascular implants, orthopedic implants, dental implants, ophthalmic implants, and others. The cardiovascular implants segment held the largest market share in 2023; it is also expected to register the highest CAGR during 2023–2031. Due to increasing research and development to develop novel cardiac implant products, the cardiovascular implants segment is expected to dominate the bio-implants market during the forecast period.
In terms of material, the bio-implants market is segmented into metals, ceramics, and polymers. The metals segment held the largest market share in 2023. The same segment is estimated to record the highest CAGR during 2023–2031. Metals are ideal for implant applications due to their remarkable mechanical strength, corrosion resistance, and biocompatibility. Titanium is an extremely popular material for orthopedic, dental, and cardiovascular implants due to its exceptional durability, lightweight design, and compatibility with human tissue.
Based on end user, the market is bifurcated into hospitals & clinics and ambulatory surgical centers. The hospitals & clinics segment is expected to register the highest CAGR during 2023–2031. The marker growth of this segment is ascribed to the growing geriatric population worldwide, resulting in a rise in health issues leading to the burgeoning demand for bio-implants as a treatment option for better health outcomes.
Bio-implants Market: Competitive Landscape and Key Developments
LifeNet Health; Smith & Nephew; Arthrex, Inc.; Clinic Lemanic; Alpha Bio Tec; MiMedx Group; Medtronic; St Jude Medical (Abbott); Stryker Cooperation; DePuy Synthes; Biomet (Zimmer); Exactech, Inc.; Cochlear Ltd; and Straumann AG are among the key companies operating in the bio-implants market. Leading players are implementing strategies such as expansions, new product launches, and acquisitions (companies or new clientele) to tap prevailing business opportunities. As per the company press releases, below are a few recent key developments:
- In April 2022, Orthopedic Implant Company received approval from the Food and Drug Administration and released the high valve dorsal scanning plate. The product helped expand the company’s orthopedic trauma portfolio for the potential clinical implantation of OIC’s DRPx Wrist Fracture Plating System, reinforcing it as one of the most comprehensive and value-driven alternatives to any other premium-priced plating systems.
- In January 2022, Johnson & Johnson Medical Devices Companies (JJMDC) collaborated with Microsoft to enhance and further develop JJMDC’s secure and compliant digital surgery ecosystem.
- In October 2021, ScottCare Cardiovascular Solutions signed an agreement with Ninety One Holding, Inc. to become the primary distributor for Ninety One’s cloud software platform for monitoring cardiac implanted devices, providing an effective expansion partnership between the two companies. This allows ScottCare Cardiocular Solutions to offer Ninety One’s cloud platform directly to clinics that work with their staff to monitor and manage patient data. Ninety One’s software platform helps in combining advanced data science with the latest technologies to help analyze and manage complex medical data for patients with implanted cardiac devices.
- In March 2020, Orthofix International N.V. acquired Spinal Kinetics Inc., a private manufacturer and developer of artificial cervical and lumbar discs. The acquisition aimed to expand the Orthofix product line of orthobiological bio-implants.