Esoteric Testing Market
Esoteric testing is performed to test various samples for rare substances or molecules, and it is not performed as a part of routine testing. This segment of diagnostics requires highly skilled professionals along with advanced and complex equipment. They are principally performed in specialized laboratories wherein samples are either referred by physicians or hospitals (inpatient testing) or by patients themselves (outpatient testing). A few of these tests are currently based on the radioimmunoassay technique, which is both expensive and time-consuming. The COVID-19 pandemic provided significant growth prospects for the esoteric testing market in the last couple of years. The RT-PCR test is a common method that is in use for the qualitative identification of SARS-CoV-2 RNA extracted from upper and lower respiratory samples (obtained from nasopharyngeal or oropharyngeal swabs, sputum, etc.) collected from persons suspected of COVID-19. The rising prevalence of chronic diseases and growing awareness of early detection of medical conditions propel the esoteric testing market growth.
Report Segmentation and Scope:
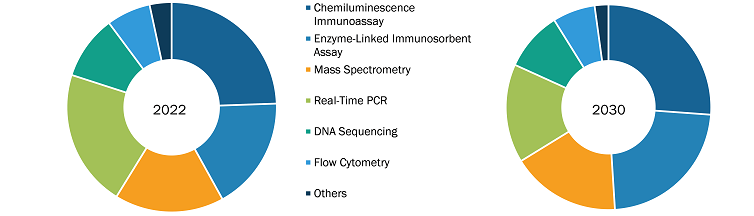
The “Esoteric Testing Market” is segmented on the basis of type, technology, and end user. Based on type, the market is segmented into infectious disease testing, endocrinology testing, oncology testing, toxicology testing, immunology testing, neurology testing, and others. In terms of technology, the market is divided into chemiluminescence immunoassay, enzyme-linked immunosorbent assay, mass spectrometry, real-time PCR, DNA sequencing, flow cytometry, and others. In terms of end user, the esoteric testing market is categorized into hospitals and laboratories, and independent and reference laboratories.

Growing Awareness of Early Detection of Medical Conditions Drives Esoteric Testing Market Growth
The early diagnosis of diseases helps medical professionals and patients to make various important medical decisions in terms of care, support needs, and financial and legal matters. Obtaining accurate and early diagnosis after noticing cognitive symptoms related to a specific medical condition results in the reversal of symptoms before they are aggravated, followed by an effective and early treatment of patients. It also provides healthcare professionals sufficient time for better decision-making. Laboratory examination procedures are widely performed for the diagnosis of diseases associated with medical specialties such as neurological disorders, genetic diseases, cancer, and endocrinological disorders. Esoteric testing and genetic testing are performed for the diagnosis of diabetes, cardiovascular disease, lipid disorders, cancer, and genetic diseases.
Various government regulatory bodies are also taking initiatives and conducting campaigns to promote early diagnosis practices. The National Organization for Rare Disorders aims to identify, diagnose, and treat rare disorders through numerous supportive programs, including education, research, advocacy, and patient services. The New York State Rare Disease Workgroup, created in response to legislation passed in New York State (Article 27-L of the Public Health Law), aims to identify best practices that could enhance public awareness of rare diseases. Through such initiatives, people potentially having rare diseases can be referred to specialists, which would help overcome barriers to their treatments, including financial challenges.
The government of India launched the National Policy for Rare Diseases (NPRD) in March 2021 to treat rare disease patients. This policy has a provision for financial support of up to US$ 5 million to provide medical support to patients suffering from any rare diseases by providing treatments in any of the Centre of Excellence (CoE) mentioned in NPRD-2021, outside the Umbrella Scheme of Rashtriya Arogaya Nidhi. The NPRD 2021 has provisions for encouraging research and development to diagnose and treat rare diseases, promote local development and manufacturing of drugs, and create a conducive environment for indigenous manufacturing of drugs for rare diseases at affordable prices.
Such initiatives taken to build knowledge regarding available diagnosis options for complex genetic and non-genetic rare disorders favor the esoteric testing market growth.
Strategic Initiatives Provide Growth Opportunities for Esoteric Testing Market Players
Companies operating in the esoteric testing market focus on strategic developments such as collaborations, expansions, agreements, partnerships, and new product launches, which help them improve their sales, expand their geographic reach, and enhance their capacities to cater to a larger than existing customer base. A few of the noteworthy developments in the esoteric testing market are mentioned below.
• In February 2021, Quest Diagnostics collaborated with Grail for its Galleri multi-cancer blood tests. With this collaboration, Quest Diagnostics had plans to provide phlebotomy services using the Galleri multi-cancer early-detection blood tests.
• In January 2021, BioReference Laboratories, Inc., an OPKO Health (OPK) company, introduced Scarlet Health, an in-home, fully integrated digital platform providing access to on-demand diagnostic services. Scarlet has been designed similarly to tools consumers use daily to confer ease of use, ubiquity, and convenience. The platform delivers an innovative, flexible, mobile alternative to traditional patient service centers or other draw locations when phlebotomy and other specimen collection services are needed.
• In September 2021, LabCorp acquired operating assets and intellectual property (IP) from Myriad Genetics’ Autoimmune Business Unit, including the Vectra rheumatoid arthritis (RA) assay, which is expected to bolster its scientific leadership in rheumatology.
• In 2020, Quest Diagnostics acquired Blueprint Genetics, the outreach laboratory service business of Memorial Hermann Health System. Via this multiyear agreement, Quest plans to offer professional laboratory management services for all 21 hospital laboratories of Memorial Hermann, which provide onsite rapid-response testing. Quest also plans to become the sole chosen business providing laboratory services for the Memorial Hermann Health Plan.
• In 2019, Sonic Healthcare acquired Aurora Diagnostics to augment its presence in the anatomical pathology specialty in the US.
Therefore, initiatives by companies to venture into new business segments through collaborations and partnerships to remain competitive would create significant growth opportunities in the esoteric testing market in the coming years.
Esoteric Testing Market: Competitive Landscape and Key Developments
Georgia Esoteric & Molecular Laboratory LLC, Laboratory Corp of America Holdings, Quest Diagnostics Inc., National Medical Services Inc., OPKO Health Inc., ARUP Laboratories Inc., bioMONTR Labs, Athena Esoterix LLC, Stanford Hospital & Clinics, and Foundation Medicine Inc. are a few of the key companies operating in the esoteric testing market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the market.