Small Cell Lung Cancer (SCLC) Treatment Market
Combination Therapy for Small Cell Lung Cancer Treatment Market
Combination therapy for lung cancer treatment is the latest advancement in lung cancer research, with clinical studies revealing positive results in the overall survival rate for SCLC-suffering patients. Chemotherapy drugs in combination with immunotherapy are one such example of combination therapy intended for SCLC. Atezolizumab (Tecentriq) is an FDA-approved treatment for SCLC used in combination with “carboplatin and etoposide” as a first-line therapy for adults suffering from extensive-stage SCLC. Likewise, clinical researchers have identified a combination of two drugs that results in reducing the mortality rate of SCLC-suffering patients, which is the most aggressive form of lung cancer. With the majority of patients responding positively to SCLC as a first-line treatment through chemotherapy immunotherapy, there are high chances of cancer reoccurring. Therefore, combination therapy, such as chemotherapy drugs, namely, “Topotecan (Hycamtin)” and investigational drug “Berzosertib,” is responsible for inhibiting a protein that helps repair damaged DNA caused by SCLC. The factors mentioned above are responsible for the exponential market growth during 2020–2030.
Market Opportunity
Product Launches and Fast Product Approvals
The majority of people with SCLC respond to initial treatment with chemotherapy and immunotherapy. However, cancer usually progresses despite additional treatment, and most of the patients die within weeks or months. Therefore, the fast product approvals will provide a lucrative opportunity for the market to grow exponentially from 2020 to 2030. For instance, in March 2020, the Food and Drug Administration (FDA) announced approval for “Durvalumab” with a combination of “Etoposide” and either “Carboplatin” or “Cisplastin” as a first-line treatment for patients suffering from extensive-stage SCLC. Additionally, in March 2019, the USFDA approved “Tecentriq” to treat SCLC. The newly approved “Tecentriq” is the second immunotherapy drug approved for people with advanced SCLC and a first-line treatment drug.
Small Cell Lung Cancer (SCLC) Treatment Market: Segmental Overview
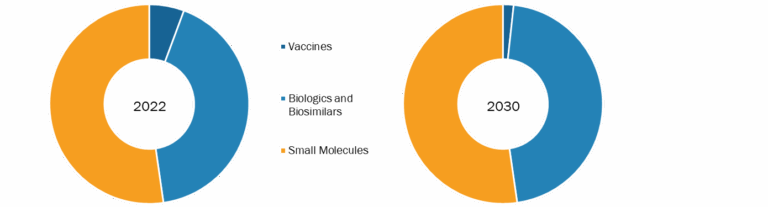
The global SCLC treatment market is segmented based on treatment, stage, and end user. Based on treatment, the market is categorized into chemotherapy, radiation therapy, immunotherapy, and others. In terms of stages, the SCLC treatment market is bifurcated into extensive stage and limited stages. The SCLC treatment market, by end-user, is segregated into hospitals, specialty clinics, homecare settings, and others. The SCLC treatment market, based on geography, is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Spain, and the Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and the Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America). Based on treatment, the SCLC treatment market is segmented into chemotherapy, radiation therapy, immunotherapy, and others. In 2022, the chemotherapy segment held the largest share of the market. The SCLC treatment market based on stage is bifurcated into extensive stage and limited stage. In 2022, the extensive stage segment held the largest share of the market. The same segment is expected to register the highest CAGR in the market during 2022–2030. Based on end users, the SCLC treatment market is segmented into hospitals, specialty clinics, homecare settings, and others. In 2022, the hospitals segment held the largest share of the market. However, the specialty clinics segment is expected to register the fastest CAGR during 2022–2030.
Small Cell Lung Cancer (SCLC) Treatment Market: Competitive Landscape and Key Developments
F. Hoffmann-La Roche, Bristol Myers Squibb, AstraZeneca, Jazz Pharmaceuticals plc, Merck KgaA, Dr. Reddy’s Laboratories, Inc., GlaxoSmithKline (GSK), Pfizer, Regeneron, and Janssen are among the leading companies operating in the SCLC treatment market. These players focus on expanding and diversifying their market presence and acquiring a novel customer base, tapping business opportunities prevailing in the SCLC treatment market. Many market players are launching innovative products with advanced features.
In November 2022, Regeneron Pharmaceuticals, Inc. announced approval for the USFDA’s “PD-1 inhibitor “Libtayo (cemiplimab-rwlc)” in combination with platinum-based chemotherapy intended for the first-line treatment of adult patients. Libtayo is also approved for extending the survival of patients suffering from advanced SCLC.
In June 2020, Jazz Pharmaceuticals plc announced USFDA approval for Zepzelcaa” to treat adult patients suffering from metastatic SCLC with disease progression on or after platinum-based chemotherapy. “Zepzelcaa” is now commercially available in the US and will drive market growth from 2020 to 2030.
In July 2021, AstraZeneca announced approval for “Imfinzi (durvalumab)” in China as a first-line treatment for all adult patients suffering from extensive-stage SCLC. The approval by China’s National Medical Products Administration was based on positive results from the CASPIAN Phase III trial, and the clinical trial revealed that “Imfinzi plus” chemotherapy demonstrated a statistically significant and clinically meaningful improvement in the survival rate of patients compared to chemotherapy alone. “Imfinzi Plus” is also being tested following concurrent chemoradiation therapy in patients suffering from limited-stage SCLC in the ADRIATIC Phase III trial as a part of a broad development program.