Stomach cancer or gastric cancer is a type of malignant cancer that spreads on the stomach’s inner lining. Age, diet, and stomach diseases are the major risk factors for gastric cancer. The indications of gastric cancer include indigestion, heartburn, bloating, and stomach discomfort or pain. The occurrence of a huge patient population with gastrointestinal tumors and adenocarcinoma, an upsurge in the geriatric population, and an increase in alcohol consumption and smoking are some other factors contributing to the growing stomach cancer prevalence, thereby positively impacting the stomach cancer market. The rise in the prevalence of stomach cancer and increasing clinical trials for the development of innovative products are among the major factors contributing to the growing stomach cancer market size.
Rising Burden of Stomach Cancer to Drive Stomach Cancer Market Growth
Stomach cancer, also known as gastric cancer, is regarded as one of the major health challenges worldwide. Stomach is lined with a mucous membrane composed of cells and glands. Before a true cancer develops, pre-cancerous changes occur in the inner lining of the stomach. The cells are prone to inflammation, leading to peptic ulcers, which could potentially become gastric cancer. The early signs of gastric cancer include nausea, indigestion, loss of appetite, heartburn, and other symptoms. When stomach cancer is severe, signs such as yellowish eyes or skin, stomach pain, swelling in the stomach, blood in stool, vomiting, constipation, and weight loss can be detected. Regardless of declining incidence and mortality during the last decades, stomach cancer is considered one of the major health issues worldwide.
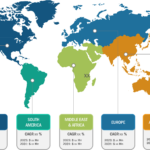
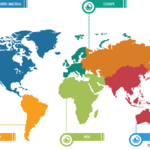
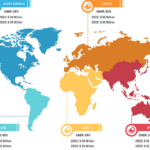
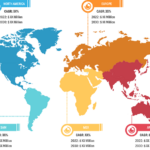
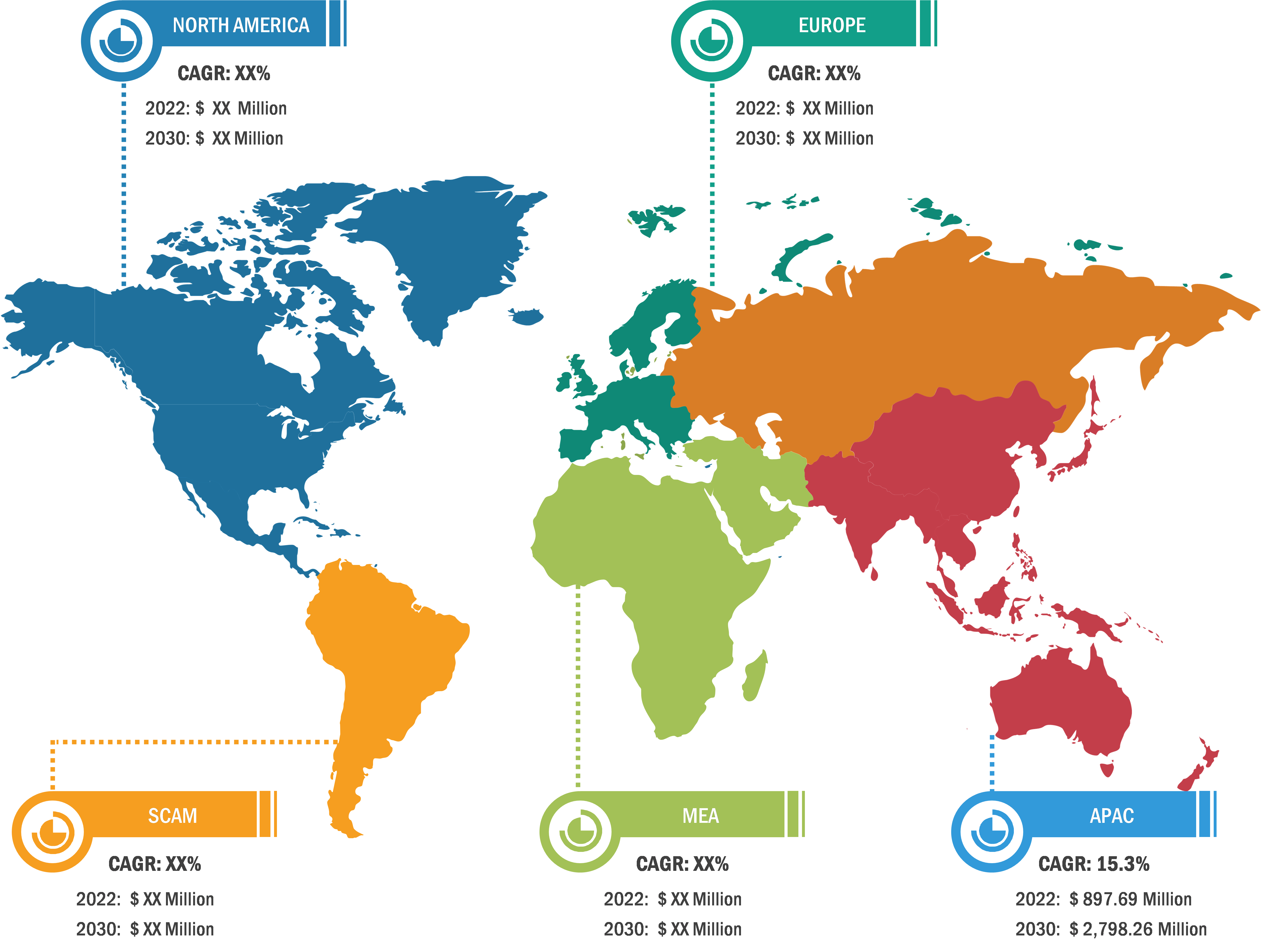
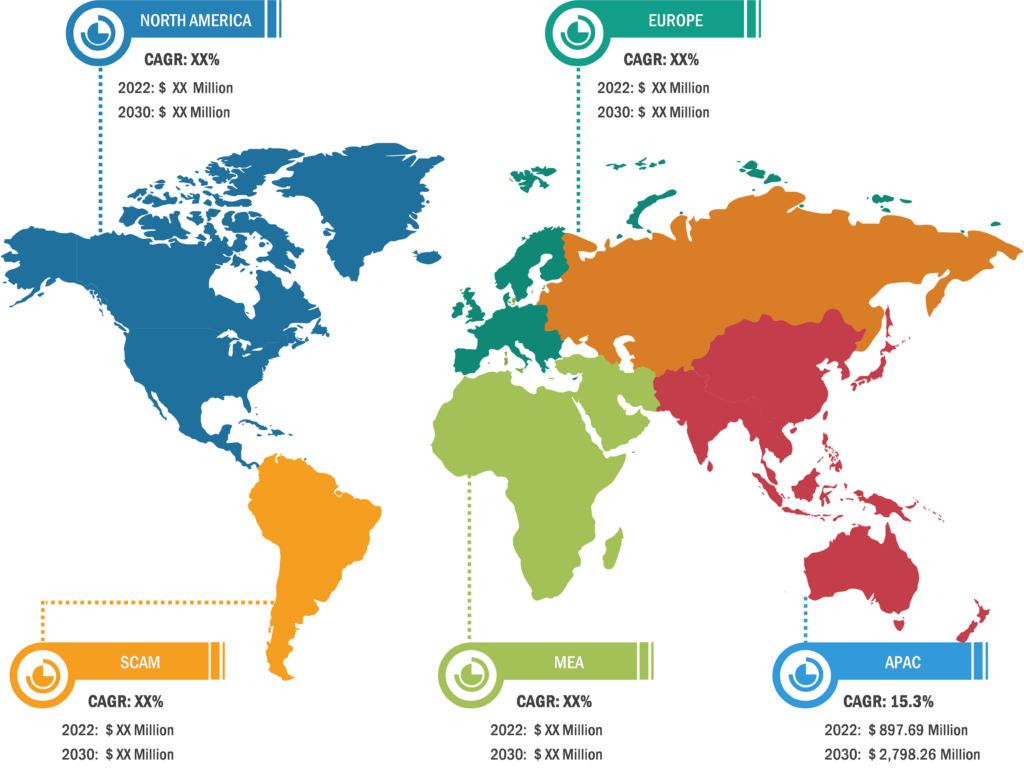
Gastric cancer is the 5th most common cancer worldwide, and it was 4th leading cause of cancer mortality in both genders combined in 2020. As per Global Cancer Statistics 2020: GLOBOCAN estimates, in 2020, ~1.1 million cases (720,000 males and 370,000 females) of gastric cancer were diagnosed worldwide, accounting for 5.6% of all cancer cases. Stomach cancer caused ~800,000 deaths (representing 7.7% of all cancer-related deaths) in 2020. Eastern Asian and Eastern European regions showed the highest incidence rates in both males and females, about two-thirds of all cases occurred in men. Of all stomach cancer cases, 60% were observed in Eastern Asia and 43.9% in China alone. It was more commonly diagnosed in males, with 66% of all instances occurring in them.
Stomach cancer accounts for 1.5% of all new cancers diagnosed in the US each year. The American Cancer Society estimates that in 2023, ~26,500 new cases (15,930 in men and 10,570 in women) of stomach cancer will occur in the US. In the same year, 11,130 deaths can happen due to this type of cancer (6,690 men and 4,440 women). Researchers from the International Agency for Research on Cancer (IARC) and collaborators predict that the annual burden of gastric cancer will upsurge to about 1.8 million new cases and about 1.3 million deaths by 2040, representing an increase of about 63% and 66%, respectively, compared to 2020.
Thus, the increasing number of cases of stomach cancer fuels the need for different types of treatment among people, driving the growth of the stomach cancer market.
Stomach Cancer Market: Segmental Overview
The stomach cancer market is segmented on the basis of type, treatment, route of administration, and distribution channel. Based on type, the stomach cancer market has been segmented into adenocarcinomas, gastrointestinal stromal tumors, neuroendocrine tumors, lymphoma, and others. The adenocarcinomas segment held a significant share of the market in 2022 and is expected to register the highest CAGR during 2022–2030. Adenocarcinoma is a type of cancer that develops from the gland cells in the innermost lining (i.e., mucosa) of the stomach. Many organs possess these types of cells, and it can develop in any of these organs. In some instances, adenocarcinoma spreads to other parts of the body and is called metastatic adenocarcinoma. Most cancers of the stomach (about 90% to 95%) are adenocarcinomas. Risk factors for adenocarcinoma vary depending on cancer type. Owing to the increasing prevalence of gastric adenocarcinoma and the rapid adoption of targeted therapy and immuno-checkpoint inhibitors for treating gastric adenocarcinoma, the segment is expected to fuel the stomach cancer market growth during the forecast period.
Stomach Cancer Market: Competitive Landscape and Key Developments
Bristol Myers Squibb Company; Novartis AG; Merck & Co., Inc.; Eli Lilly and Company; Biocon; Teva Pharmaceutical Industries Ltd; Celltrion Healthcare Co., Ltd; Samsung Bioepis; Pfizer Inc.; and Ipsen Pharma are a few of the key companies operating in the stomach cancer market. These companies focus on product innovation strategies to meet evolving customer demands, along with maintaining their brand name.
A few of the recent developments in the global stomach cancer market are mentioned below:
- In February 2023, CARsgen Therapeutics announced a collaboration agreement with F. Hoffmann-La Roche to assess AB011 and atezolizumab to treat gastric cancer.
- In November 2022, Organon Canada introduced Ontruzant, a biosimilar of the reference biologic Herceptin, providing patients with certain breast and gastric cancers with an additional therapeutic option at a lower cost in Canada.
- In October 2021, Agilent Technologies Inc. received CE-IVD mark approval for the PD-L1 IHC 28-8 pharmDx to offer options for the first-line treatment of adult patients with HER2-negative advanced or metastatic gastric, gastroesophageal junction, or esophageal cancers.
- In October 2021, the European Commission approved Bristol Myers Squibb’s Opdivo (nivolumab) in combination with fluoropyrimidine- and platinum-based combination chemotherapy for the treatment of gastrointestinal junction cancer and gastric cancer.
- In April 2021, Bristol Myers Squibb received US Food and Drug Administration (FDA) approval for Opdivo (nivolumab) in combination with fluoropyrimidine- and platinum-containing chemotherapy as the first immunotherapy for first-line treatment of patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.
- In August 2020, Samsung Bioepis Co., Ltd. launched its first oncology treatment, ONTRUZANT (trastuzumab), in Brazil. ONTRUZANT was approved by Agência Nacional de Vigilância Sanitária (ANVISA), the country’s health regulatory agency, for treating patients with metastatic HER2-overexpressing breast cancer, early HER2-overexpressing breast cancer, and advanced gastric cancer.