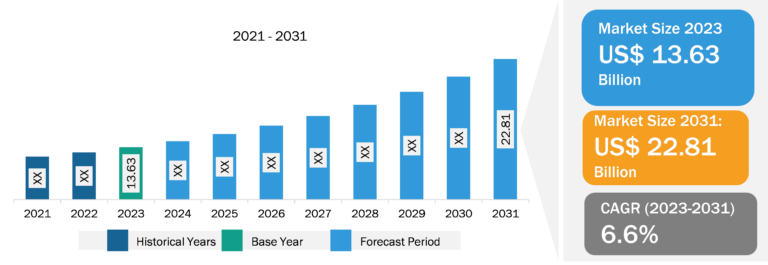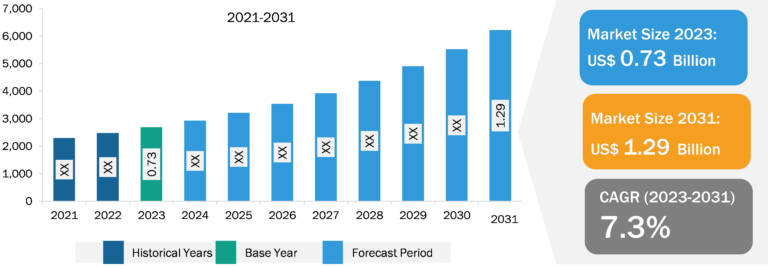
Ovarian Cancer Drugs Market
Rising Incidence of Ovarian Cancer Propels Demand for Therapeutic Options
Ovarian cancer is the seventh most common cancer among women and the third most common gynecological malignancy, after cervical cancer and endometrial (uterine) cancers, respectively. According to the World Ovarian Cancer Coalition report, 324,000 women are diagnosed with ovarian cancer, and 207,000 women die of the disease annually. Further, the majority of patients with ovarian cancer are diagnosed with an advanced-stage (locally advanced or metastatic) condition as there is no public health screening program available for the early detection of ovarian cancer. Therefore, the rising incidence of ovarian cancer propels the demand for effective therapeutic options. The first-line therapy with a combination of debulking surgery and platinum-based chemotherapy is standard care for women newly diagnosed with advanced ovarian cancer. Also, therapeutic options such as approved PARP inhibitors, olaparib, rucaparib, and niraparib are the breakthrough options for the management of newly diagnosed ovarian cancer. The use of PARP inhibitors in the management of advanced ovarian cancer has also emerged as an effective therapeutic option to improve clinical outcomes. These inhibitors provide long-term efficacy and progression-free survival (PFS) in the newly diagnosed cases, following a complete response (CR) to the first, second, and third platinum-based chemotherapy. Therefore, significant product approvals for the ovarian cancer treatment drugs will have a positive impact on the ovarian cancer drugs market size.
Therefore, the aforementioned factors are increasing the demand for ovarian cancer drugs for the disease management, facilitating the ovarian cancer drugs market share expansion.
Ovarian Cancer Drugs Market Trends
Combination Drug Therapy
Several medical research institutes are developing novel combination drugs to treat ovarian cancers. In September 2023, researchers at the Royal Marsden announced a new drug combination therapy that proves effective in patients living with advanced ovarian cancer. The researchers tested combination drug therapy for its effect on low-grade serous ovarian cancer (LGSOC), a rare form of ovarian cancer with a poor response rate to treatments. The RAMP-201 clinical trial was conducted by combining the avutometinib drug with defactinib to investigate their synergistic therapeutic effect in patients suffering from LGSOC. The interim clinical trial results reveal that 45% of the patients treated with the drug combination saw their tumors shrink significantly. Such satisfactory clinical results with combination therapy are almost twice as effective compared to trametinib, a targeted therapy drug available in England that has a response rate of only 26%. In January 2024, the FDA announced approval for the SH-105 combination drug for the treatment of patients with breast and ovarian cancer. Thus, combination drug therapies are emerging as new trends that are likely to boost the ovarian cancer drugs market growth in the coming years.
Ovarian Cancer Drugs Market: Geographic Overview
The ovarian cancer drugs market, by geography, is segmented North America (US, Canada, and Mexico), Europe (Spain, UK, Germany, France, Italy, and Rest of Europe), Asia Pacific (South Korea, China, India, Japan, Australia, and Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, the UAE, and Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and Rest of South & Central America). In terms of revenue, North America dominated the market in 2022. Among North America region, US holds largest share for ovarian cancer drugs market. Rising incidence of ovarian cancer among US women and fast drug approvals for ovarian cancer treatment are the influential factors responsible for ovarian cancer market growth. Also, contract clinical research organizations (CROs) by the US manufacturers is responsible for influential ovarian cancer drugs market growth for the forecast period. According to a report by the US Department of Health and Human Services, ~235,511 women were living with ovarian cancer in the US in 2020. Additionally, the American Cancer Society estimates that ~19,680 women in the US would be newly diagnosed with ovarian cancer in 2024, while nearly 12,740 patients are likely to die from the disease. In April 2020, the FDA announced approval for Niraparib (Zejula) manufactured by GSK for the treatment of epithelial ovarian cancer patients who completely or partially respond to the first-line platinum-based chemotherapy.
Ovarian Cancer Drugs Industry Developments and Future Opportunities:
A few strategic developments by leading ovarian cancer drugs market players, as per company press releases, are listed below:
- In September 2022, AstraZeneca and Merck received approval for Lynparza (olaparib) in China for the maintenance treatment of adult patients suffering from advanced epithelial cancer. The newly approved candidate is the first-line platinum-based chemotherapy administered in combination with bevacizumab.
- In November 2022, ImmunoGen won FDA approval for ELAHERE (mirvetuximab soravtansine-gynx) for the treatment of adult patients resistant to platinum-resistant epithelial ovarian cancer. The ELAHERE is specifically approved for patients with FRα-positive platinum-resistant ovarian cancer, which is a challenging condition due to the availability of limited treatment options and poor clinical outcomes of the existing therapeutics.
Ovarian Cancer Drugs Market: Competitive Landscape and Key Developments
The ovarian cancer drugs market analysis is carried out by identifying and evaluating key players in the market across different regions. Elli Lilly, AstraZeneca, GSK, Zielab, ImmunoGen (AbbVie), GeneTech (Roche), Vivesto, Allarity Therapeutics, Inc., Aeterna Zentaris, and Luye Pharma are among the prominent players profiled in the ovarian cancer drugs market report. These companies focus on introducing new technologies, upgrading existing products, and undertaking geographic expansions to address the globally increasing consumer demand.