Dry Eye Products Market
Rising Prevalence of Dry Eye Bolsters Dry Eye Products Market
Dry eye disease (DED) ranks third among the most common reasons for visits to an ophthalmology clinic, after refractive errors and cataracts, respectively. The prevalence of dry eye is particularly rising in developed nations. Dry eye is caused by multiple factors that result in discomfort, irritation, and visual disturbance. According to an article published by the American Academy of Opthalmology, DED affects approximately 1 in 11 people worldwide, with prevalence ranging from 5% to 50% at various ages. According to data released by Alcon in 2022, the disease affects ~719 million people globally and ~38 million people in the US. Moreover, the increasing use of digital screens can be associated with a rise in computer vision syndrome (digital eye strain) cases, affecting 26–70% of screen users globally. The prevalence of DED is higher in women and the Asian population among the major demographic groups.
Progressive damage caused to the retina due to diabetic retinopathy further results in DED. According to an article published by the American Academy of Ophthalmology, ~191.0 million people globally are estimated to suffer from diabetic retinopathy by 2030. As per the Centers for Disease Control and Prevention (CDC), approximately 9.6 million people (across all ages) in the US were living with diabetic retinopathy in 2021. Thus, the increasing incidence of diabetic retinopathy is driving the growth of the dry eye products market.
Dry Eye Products Market: Regional Overview
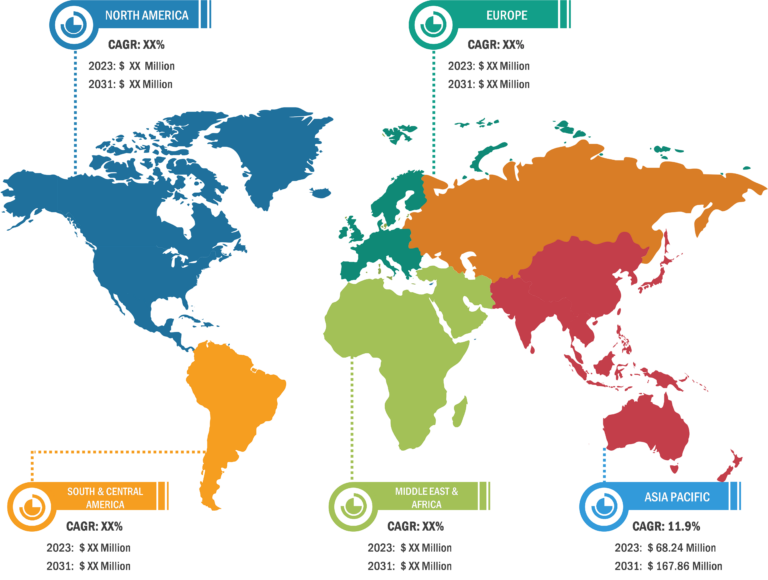
In terms of geography, the dry eye products market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2023, North America held the largest market share. The market growth in North America can be mainly associated with the increasing awareness of DED, the growing aging population, technological advancements, and rising eye disease cases. Moreover, the easy availability of various drugs due to frequent launches of new drugs is one of the key factors propelling the dry eye products market growth in the region.
DED is a common but underdiagnosed condition in the US, and an increase in the population age can be associated with the surging prevalence of this disease. According to the Centers for Disease Control and Prevention (CDC), age-related eye diseases such as cataract, macular degeneration, diabetic retinopathy, and glaucoma are the primary causes of blindness and impaired vision in the US. Other common eye disorders include amblyopia and strabismus. Dry eye disease is expected to affect as many as 2.79 million US men by 2030. Thus, with the rising incidence of dry eye disease, the demand for therapeutic products is on the rise in the country. In January 2021, Kala Pharmaceuticals Inc. launched EYSUVIS 0.25% (loteprednol etabonate ophthalmic suspension) for the short-term (up to two weeks) treatment of dry eye disease symptoms. EYSUVIS is now available in national and regional pharmaceutical distribution centers across the US.The market in Asia Pacific is attributed to the increasing incidence of DED among young people due to prolonged usage of computers, growing government support for eye health, and ongoing developments in ophthalmic solutions. According to NIH, in China, ~20–30% of people are diagnosed with dry eye conditions every year. The dry eye products market in China is quite competitive, with the presence of both domestic and international pharmaceutical companies. Novaliq has established a strategic cooperation with Jiangsu Hengrui Pharmaceuticals to manufacture, develop, and commercialize NOV03 for the treatment of dry eye disease. In December 2021, Hengrui obtained positive outcomes from the critical Phase 3 clinical trial of SHR8058 (NOV03) for the treatment of dry eye disease linked with meibomian gland dysfunction; the trial was conducted at 17 sites across China. The Chinese firm is expected to distribute these products across China and Southeast Asia. Thus, the contributions and market initiatives of the Novaliq and Hengrui in China are propelling the dry eye products market growth.
Dry Eye Products Market: Competitive Landscape and Key Developments
Apart from factors driving the market, the dry eye products market report emphasizes prominent players operating in the market; these include Santen Pharmaceutical Co Ltd, Johnson & Johnson, OASIS Medical, URSAPHARM Arzneimittel GmbH, Rohto Pharmaceutical Co Ltd, OCuSOFT Inc, Bausch Health Companies Inc, AbbVie Inc, Prestige Consumer Healthcare Inc, Farmigea SpA, and Alcon AG. Market players focus on expanding and diversifying their businesses and acquiring novel customer bases, which allows them to explore attractive business opportunities prevailing in the dry eye products market. As per company press releases, a few recent developments in the dry eye products market are as follows:
• In September 2023, Bausch + Lomb Corporation (Bausch + Lomb), a subsidiary of Bausch Health Companies Inc, completed its acquisition of XIIDRA (lifitegrast ophthalmic solution) 5%, a non-steroid eye drop specifically approved to treat the signs and symptoms of dry eye disease. The product specifically focuses on inflammation associated with dry eye and certain other ophthalmology assets.
• In November 2022, Santen Pharmaceutical Co Ltd launched DIQUAS LX Ophthalmic Solution 3% (diquafosol sodium) in Japan. The DIQUAS LX solution is a formulation that requires the administration of one drop three times daily to treat dry eye disease.
• In August 2022, Alcon and Aerie Pharmaceuticals Inc entered into a definitive merger agreement. As a part of this deal, Alcon acquired Aerie. This transaction helps bolster Alcon’s presence in the ophthalmic pharmaceutical space with its growing portfolio of commercial products and development pipeline.
• In February 2021, Allergan launched Refresh Digital, a new lubricant eye drop designed to specifically relieve dryness and irritation caused by prolonged screen time.
• In January 2020, Santen Pharmaceutical Co Ltd decided to construct a second plant for its Chinese subsidiary, named Santen Pharmaceutical (China) Co Ltd., to support the growing demand for products in China. The plan was in line with its intentions to expand its business in China.