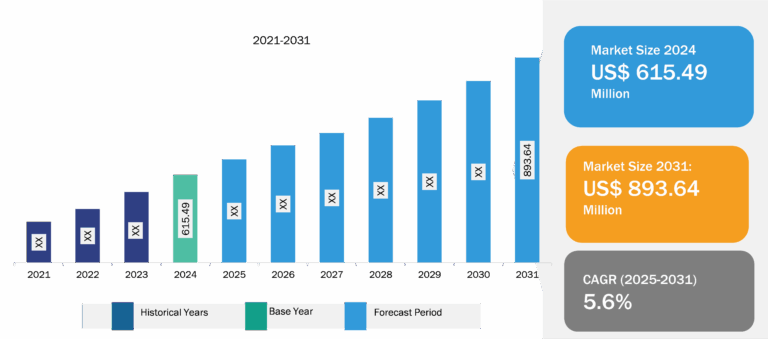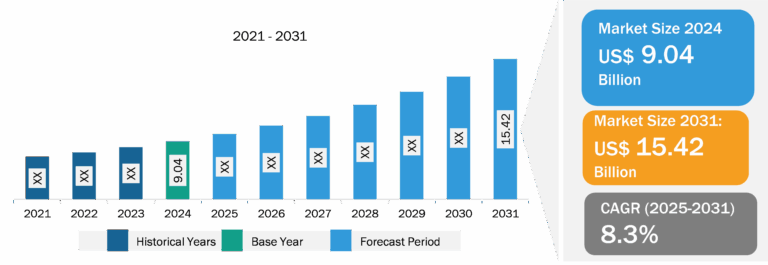
Blood Collection Tubes for Liquid Biopsy Market
The report emphasizes the trends prevalent in the global market, along with drivers and deterrents affecting its growth. An upsurge in the prevalence of cancer and the advantages of liquid biopsy over other diagnostic testing techniques are among the pivotal factors responsible for market growth. However, a lack of skilled professionals hamper the market growth. Technological advancements in liquid biopsy are expected to bring new trends in the blood collection tubes for liquid biopsy market in the coming years.
Technological Advancements in Liquid Biopsy to Act as Trend in Blood Collection Tubes for Liquid Biopsy Market During Forecast Period
Liquid biopsy has revolutionized the way cancer disease states are detected and monitored. Circulating tumor cell (CTC) tubes, intracellular RNA tubes, circulating cell-free DNA (ccfDNA) tubes, cell-free RNA (cfRNA) tubes, genomic DNA (gDNA) tubes, and more advanced blood collecting tubes are available for liquid biopsy. The ccfDNAs, cfRNAs, and extracellular vehicles (also known as exosomes) are gaining considerable attention in cancer diagnosis. Disease-specific exosome or cfRNA analyses require draw-time concentrations of these analytes preserved in collected samples. Major market players focus on introducing new technologically advanced products in the market. For instance, in November 2021, PreAnalytiX GmbH launched the QIAsymphony PAXgene Blood ccfDNA Kit (CE-IVD) to fulfill the new EU IVDR requirements. The QIAsymphony PAXgene Blood ccfDNA Kit and the PAXgene Blood ccfDNA Tube (CE-IVD) have been verified as an integrated workflow solution for reproducible and standardized ccfDNA processing in diagnostic laboratories requiring automated solutions.

In November 2023, Illumina, Inc., launched TruSight Oncology 500 ctDNA v2, a research assay that enables noninvasive comprehensive genomic profiling (CGP) of circulating tumor DNA (ctDNA) from blood when tissue testing is not feasible; the assay is also performed to complement tissue-based testing. In February 2024, Delfi Diagnostics, in collaboration with Immunocore Holdings plc, launched a new cancer monitoring test to examine blood for broken pieces of tumor DNA to explore its use in the detection of multiple malignancies. The DELFI-TF assay delivers a genome-wide measure of the proportion of cfDNA that is derived from a tumor, and it is highly correlated with the mutant allele fraction that is used to evaluate treatment response and resistance to immunotherapies in patients suffering from advanced-stage cancer conditions. Thus, technological advancements in liquid biopsies for the detection of cancer are expected to introduce new growth trends in the blood collection tubes for liquid biopsy market in the coming years.
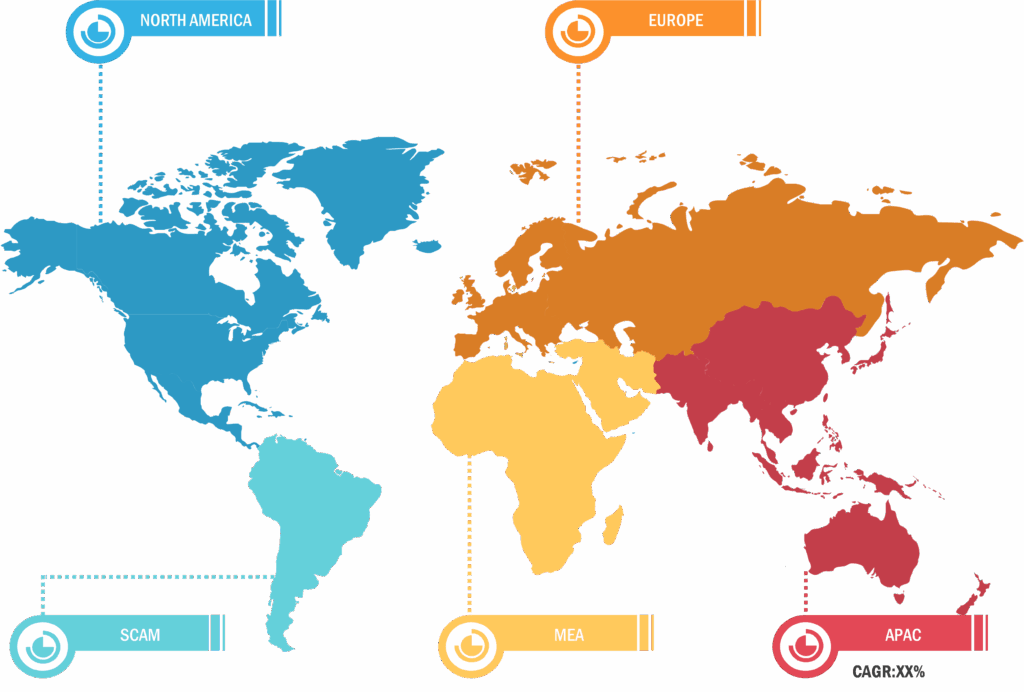
The scope of the blood collection tubes for liquid biopsy market report covers North America (US, Canada, and Mexico), Europe (Spain, UK, Germany, France, Italy, and Rest of Europe), Asia Pacific (South Korea, China, India, Japan, Australia, and Rest of Asia Pacific), the South & Central America ( Brazil, Argentina, and Rest of South & Central America), and the Middle East & Africa (South Africa, Saudi Arabia, UAE, and Rest of Middle East & Africa). North America held the largest share of the blood collection tubes for liquid biopsy market in 2023. The market for liquid biopsy procedures is flourishing in the US with the surging prevalence of cancer, high spending on the development of innovative diagnostics solutions, and the presence of significant market players in the country. The National Cancer Institute estimates that ~2 million new cases of cancer would be registered in the US in 2024, and ~611,720 people are expected to die due to cancer. As per the same source, ~440.5 per 100,000 people are diagnosed with cancer every year. Tissue biopsies are highly invasive techniques employed for lung cancer diagnosis. As the insufficiency of samples may result in nonreliable results in this type of biopsy, researchers focus on the development of liquid biopsy techniques for lung cancer diagnoses. In December 2022, Agilent Technologies Inc. received approval from the US FDA for Agilent Resolution ctDx FIRST as a companion diagnostics (CDx) solution to identify advanced non-small cell lung cancer (NSCLC) patients with KRAS G12C mutations, who may benefit from the KRAZATI (adagrasib) treatment. Such approvals from the US FDA of liquid biopsy tests would propel the demand for blood collection tubes for liquid biopsy in the US.
As per the Canadian Cancer Society’s estimates, ~2 in 5 Canadians are likely to be diagnosed with cancer in their lifetime, and ~1 in 4 Canadians is expected to die due to cancer. As per the same source, approximately 127,000 males and 120,000 females would be diagnosed with cancer in 2024. The soaring prevalence of cancer is likely to propel the demand for effective diagnostic techniques, such as liquid biopsy along with blood collection tubes, in Canada. Mounting investments by Canadian companies for the development of cancer detection kits based on liquid biopsy techniques fuel the growth of the Canadian blood collection tubes for liquid biopsy market. In July 2021, Adela, a spinout from Canada’s University Health Network, received US$ 60 million in financing for the development and commercialization of DNA methylation-based liquid biopsy technology. This technology may have applications across the spectrum of cancer testing applications, especially early detection of multi-cancer conditions.
The blood collection tubes for liquid biopsy market analysis has been carried out by considering the following segments: product, material, application, end user, and geography. Based on product, the blood collection tubes for liquid biopsy market is divided into ccfDNA tubes, cfRNA tubes, CTC tubes, gDNA tubes, intracellular RNA tubes, and others. Based on material, the blood collection tubes for liquid biopsy market is divided into glass and plastic. The global blood collection tubes for liquid biopsy market, based on application is segmented into In-Vitro Diagnostics (IVD), and research. Based on end user, the blood collection tubes for liquid biopsy market is divided into genetic diagnostic labs, R&D centers, conventional diagnostic centers, and others.
Blood Collection Tubes for Liquid Biopsy Market: Competitive Landscape and Key Developments
Biocept Inc, F. Hoffmann-La Roche Ltd; Streck Inc; Norgen Biotek Corp; Exact Sciences Corp; MagBio Genomics, Inc; Zymo Research Corporation; Apostle Sciences; QIAGEN NV; and Greiner Bio-One International GmbH are among the prominent players profiled in the blood collection tubes for liquid biopsy market report. In addition, several other players have been studied and analyzed during the study to get a holistic view of the market and its ecosystem. These companies focus on geographic expansions and new product launches to meet the increasing demand from consumers worldwide and increase their product range in specialty portfolios. Their global presence allows them to serve a large customer base, subsequently facilitating market expansion.