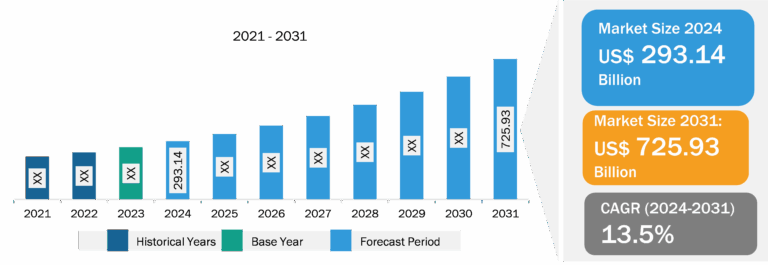
Europe Medical Device Vigilance Software Market
Rise in Medical Technology to Fuel Medical Device Vigilance Software Market Growth During Forecast Period
The medical device vigilance system’s goal is to improve the health and safety of patients, healthcare professionals, and other users by reducing the possibility of incidents related to the use of a medical device. The medical technology market comprises the market players that develop, manufacture, and distribute medical equipment, devices, diagnostic tests, and health information systems. Medical device vigilance system transforms healthcare through early disease detection, less invasive procedures, and more effective treatments. It contributes to the adoption of various types of digital solutions to carry out the efficient operation of medical devices. Medical device vigilance software helps register, track, maintain, and analyze the complete events of medical device malfunctions. With the digitalization in the healthcare industry, medical device manufacturers are adopting medical device vigilance software to comply with their ongoing post-market surveillance responsibilities.
A medical device vigilance software monitors and reports adverse events associated with the use of medical devices to detect any potential problems with medical devices and enhance their safety and effectiveness. Thus, the growing number of medical devices generates the demand for a software solution for post-market surveillance and systematic collection of information concerning medical devices’ safety use, driving the medical vigilance software market growth in Europe.

Europe Medical Device Vigilance Software Market: Industry Overview
The Medical Device Vigilance Software market is segmented on the basis of application, deployment mode, and end-use vertical. Based on application, the Europe medical device vigilance software market is segmented into diagnostic, therapeutic, surgical, research, and others. In terms of deployment mode, the market is segmented into cloud and on-premise. By end-use vertical, the medical device vigilance software market in Europe is segmented into clinical research organizations (CROs), business process outsourcing (BPO), original equipment manufacturers (OEMs), and others.
The Europe Medical Device Vigilance Software market is segmented into France, Germany, Spain, Russia, UK, and the Rest of Europe. In Europe, there has been a rising focus on the safety and security of its citizens. The medical device vigilance system’s goal is to improve the health and safety of patients, healthcare professionals, and other users by lowering the likelihood of reoccurrence of incidents related to the use of a medical device. The authorities in EU member states are in charge of enforcing the harmonized laws regarding medical devices. The regulations, such as medical device regulation (MDR) and in vitro diagnostic regulation (IVDR) in Europe, are highly focused on guaranteeing the safety of medical products. All medical devices and IVDs placed on the market are subject to the vigilance obligations outlined in EU MDR or IVDR. Under these regulations, the medical device manufacturers in Europe must understand the requirements relative to the reporting of device-related issues to competent authorities. The medical device vigilance software helps with the post-market vigilance and inspection of medical devices. This software assists in meeting regulatory requirements by automating the tracking and reporting of incidents. It includes mandatory reporting to the relevant competent authorities within pre-specified time frames. In addition, the software provides a means of reporting and tracking incidents, gathering evidence, performing analyses, and generating reports. Medical device vigilance software enhances the management of adverse events and improves transparency in post-market activities. The software also helps healthcare institutions collect real-time data that facilitate trend identification in the efficacy and safety of the device. All these benefits of the software fuel its adoption in Europe.
Several key players in the European market are engaged in the development and deployment of medical device vigilance software. Market players such as Veeva Systems and Intel offer robust solutions to cater to the requirements of the European medical device market. For example, in May 2024, CathWorks announced that the CathWorks FFRangio System is approved under the European Union (EU) Medical Device Regulation 2017/745—commonly referred to as EU MDR—and fulfills the requirements for CE marking (0344). Thus, the adoption of vigilance system to streamline adverse event reporting and regulatory compliance, enhance data management, and support risk assessment activities drives the medical device vigilance software market growth in Europe.
Europe Medical Device Vigilance Software Market: Competitive Landscape and Key Developments
Oracle Corp; AB Cube S.A.S.; Sarjen Systems Pvt. Ltd; AssurX, Inc.; UL Solutions Inc; Honeywell International Inc; PTC Inc; Intel Corp; Max Application; and Xybion Digital Inc. among others are among the leading players profiled in the Medical Device Vigilance Software market report. Several other essential market players were analyzed for a holistic view of the market and its ecosystem. The Medical Device Vigilance Software market report provides detailed market insights, which help the key players strategize their market growth. As per the company press releases, a few key developments are mentioned below:
- Honeywell announced the launch of Honeywell Quality Management Review (HQMR), a new application designed to significantly improve oversight of quality management systems (QMS) in the life sciences industry. By digitalizing and optimizing operations to increase productivity, the application supports Honeywell’s alignment of its portfolio to three compelling megatrends, including automation. The traditional Quality Management Review (QMR) process is essential for regulatory compliance in the life sciences industry, but it can often be time-consuming, costly and labor-intensive. To streamline the QMR process, Honeywell developed HQMR by removing inefficiencies and delivering near real-time intelligent insights for management. (Source: Honeywell International, Press Release, August 2024)
- AssurX, Inc. announced the availability of its Manufacturing Incident Report (MIR) Solution for EU MDR incident report automation. The AssurX solution aligns business logic with the most up-to-date MIR template published by the European Commission (EC). The solution helps ensure information accuracy, timeliness of submissions, and full transparency. The AssurX EU MDR solution enables medical device manufacturers to automate MIR documentation and submission in accordance with vigilance guidelines and reporting time frames. (Source: AssurX, Inc., Press Release, February 2020)