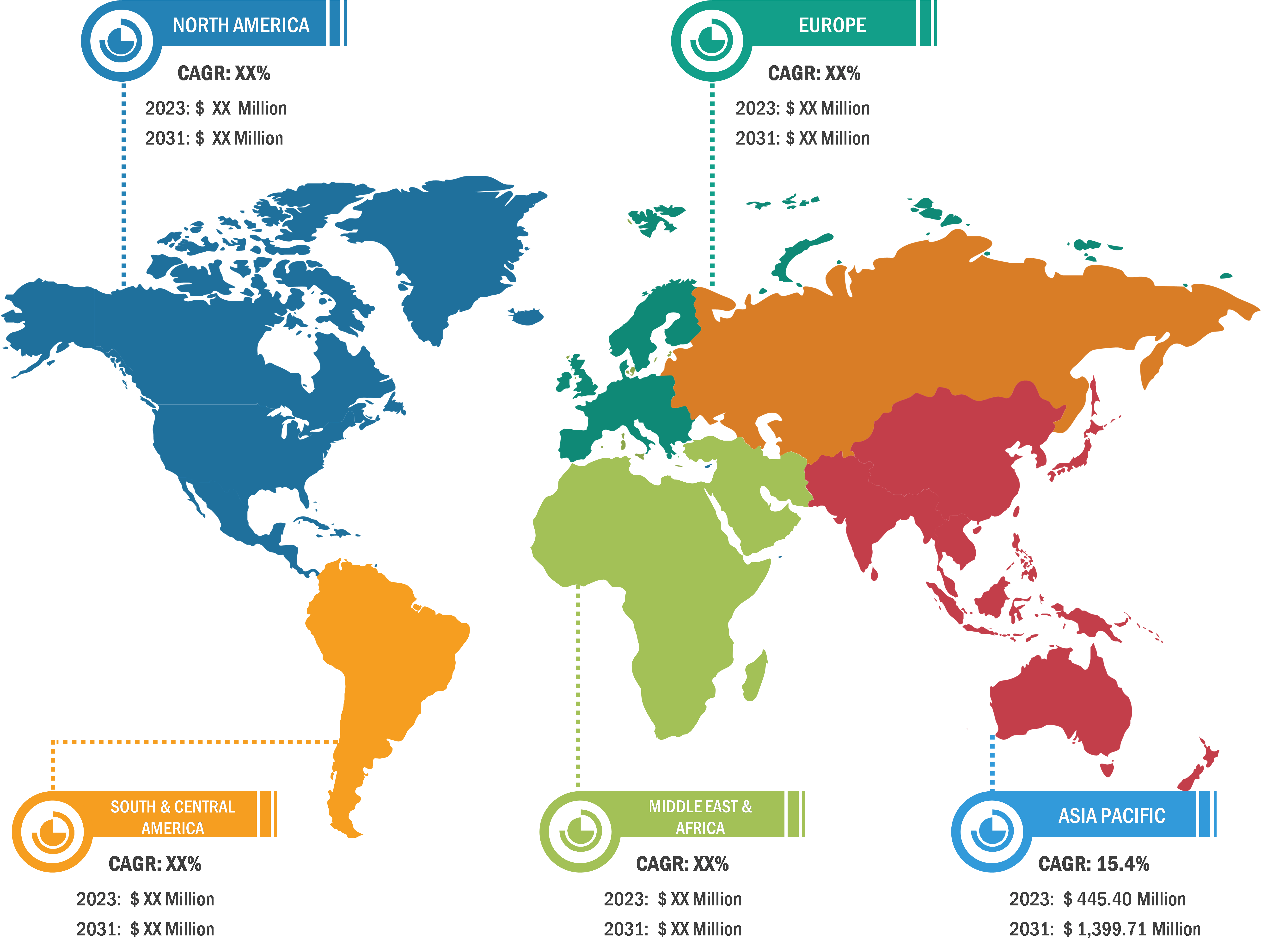
Neurorehabilitation Devices Market
Neurorehabilitation devices are specially designed to assist individuals recovering from neurological injuries or illnesses, such as stroke, traumatic brain injury (TBI), or Parkinson’s disease. These devices utilize advanced technologies, such as robotics, brain-computer interfaces, and noninvasive brain stimulation, to enhance rehabilitation outcomes. Their primary usage includes facilitating motor recovery, improving cognitive functions, and promoting neuroplasticity. Brain-computer interfaces are a significant advancement in neurorehabilitation, allowing direct communication between the brain and external devices. They are particularly effective for patients with severe motor impairments, enabling them to control assistive devices or even robotic limbs through thought alone. This technology is being explored extensively for stroke rehabilitation, where it can help patients regain motor functions by facilitating brain plasticity and retraining motor pathways. Additionally, these devices are an essential component of personalized therapy, enhancing the recovery process and enhancing patients’ quality of life. In addition, the use of smart technologies and mobile applications also supports cognitive rehabilitation, helping patients manage daily tasks and improve their cognitive abilities. Overall, the combination of innovative neurorehabilitation devices and tailored therapeutic approaches is essential for optimizing recovery and enhancing the quality of life for TBI patients. As awareness of the benefits of early and personalized rehabilitation grows, healthcare providers are increasingly adopting these innovative technologies. Additionally, advancements in electromechanical and robotic devices are further influencing market dynamics, enhancing treatment efficacy and patient engagement in rehabilitation programs. The increasing prevalence of neurological diseases worldwide and the growing number of clinical studies on neurorehabilitation devices are noteworthy factors contributing to the growing neurorehabilitation devices market size. However, the high cost of neurorehabilitation devices hinders market growth. Moreover, technological advancements in neurorehabilitation devices are expected to bring new neurorehabilitation devices market trends in the coming years.
Rising Prevalence of Neurological Diseases Fuels Neurorehabilitation Devices Market Growth
Patients suffering from neurological diseases such as stroke, Parkinson’s disease, multiple sclerosis (MS), Alzheimer’s disease, and cerebral palsy require neurorehabilitation devices to improve their motor skills, cognitive abilities, and overall quality of life.
Stroke is a primary cause of long-term disability and reduces mobility in half of stroke survivors aged 65 and older. As per the Global Stroke Fact Sheet 2022, every year, there are more than 12.2 million new strokes. One in four people over 25 are likely to have a stroke in their lifetime. The factsheet also stated that there are more than 101 million people currently living across the globe who have already experienced a stroke. The Centers for Disease Control and Prevention projected that annually, more than 795,000 people in the US have a stroke. Neurorehabilitation devices are widely used to improve memory and communication difficulties and reduce neuropsychiatric symptoms, thereby helping improve the quality of life of dementia patients. Per the World Health Organization fact sheet, in 2023, globally, more than 55 million people have dementia, and ~10 million new cases are diagnosed every year.
As per the Parkinson’s Foundation, in 2022, approximately one million people in the US had Parkinson’s disease, and ~90,000 new cases are diagnosed every year. The number of patients who have Parkinson’s disease is expected to rise to 1.2 million by 2030.
The increasing prevalence of neurological diseases worldwide creates a burden on healthcare systems, bolstering the demand for neurorehabilitation devices, and thereby fueling the neurorehabilitation devices market growth.
Neurorehabilitation Devices Market: Competitive Landscape and Key Developments
Blackrock Microsystems Inc, Hocoma AG, Medtronic Plc, Tyromotion GmbH, Ekso Bionics Holdings Inc, BIONIK, Abbott Laboratories, Renishaw Plc, EMOTIV, and BioXtreme Ltd. are a few key companies operating in the market. These companies focus on product innovation strategies to meet evolving customer demands, along with maintaining their brand name in the neurorehabilitation devices market.
As per company press releases, a few recent developments initiated in the neurorehabilitation devices market report are mentioned below:
- In August 2024, the Indian Institute of Technology (IIT) Madras and Christian Medical College (CMC), Vellore, created a low-cost, portable robotic device that can be used to provide stroke sufferers with physical therapy for their hands. Pluto (Plug and train robot for hand neurorehabilitation) is a gadget that draws inspiration from the design of a mixer grinder, which has many attachments driven by a single motor.
- In December 2022, Ekso Bionics acquired the Human Motion and Control business unit from Parker Hannifin Corporation, a global leader in motion and control technologies. The acquisition includes the Indego lower limb exoskeleton line of products and the development of robotic-assisted orthotic and prosthetic devices. The acquisition expands Ekso’s product offering across the continuum of care to home and community use markets, expands the company’s product pipeline, and enhances strategic connections with key commercial and research partners.
- In June 2022, per a partnership agreement between Blackrock Neurotech and the University of Pittsburgh’s Rehab Neural Engineering Labs, brain-computer interface (BCI) trials became more accessible to a greater population of candidates living with paralysis. The agreement sets the stage for Pitt RNEL to undertake future brain-computer interface (BCI) studies, enabling more individuals with remote BCI systems to participate in study sessions from their homes.
- In April 2022, Blackrock Neurotech partnered with Phantom Neuro to carry out research and development for Phantom Neuro’s patent-pending Phantom X system, which offers patients highly accurate, nearly real-time control of current and next-generation assistive devices, including prosthetics and exoskeletons.
- In November 2021, Blackrock Neurotech received Breakthrough Device designation for its groundbreaking MoveAgain BCI System from the US Food and Drug Administration (FDA). The first-of-its-kind system is expected to provide immobile patients with the ability to control a mouse cursor, keyboard, mobile device/tablet, wheelchair, or prosthetic device simply by thinking.