Radiopharmaceuticals Market
Nuclear medicine is a field that is undergoing many changes. As radiopharmaceuticals evolve rapidly, more advanced diagnostic techniques such as PET and radiotherapeutics are becoming more widely used. In low-income countries, the technology is still being established, owing to the efforts by the International Atomic Energy Agency (IAEA), while in some of the technologically advanced countries such as the US, it is evolving into standard care, particularly with the advent of theranostics.
Several African countries are working toward expanding and modernizing their radiopharmaceutical facilities to produce radiopharmaceutical medicines with the help of IAEA’s Technical Cooperation Program and coordinated research projects. For instance, with IAEA’s support, Tunisia, a country in North Africa, started using PET. At the same time, in Algeria, a medical cyclotron was installed and commissioned. The machine enables the country to produce its radiopharmaceuticals and enables routine PET imaging for many types of cancer, such as lymphoma and lung and colon cancer.
The International Symposium on Trends in Radiopharmaceuticals showcases the bright future of radiopharmaceutical chemistry and nuclear medicine. Advances in isotope production, radiochemistry, and the implementation of novel radiopharmaceuticals in human studies lead to exciting results. In particular, theranostic strategies, which use imaging to guide targeted therapy, and the growing areas of research are helping the global nuclear medicine community realize the potential of personalized medicine through the development of new radiotherapeutics. Theranostics merges diagnostics and therapy in a single approach. Radioactive tracers identify specific targets (diagnosis) and then perform targeted therapies. This approach lets physicians personalize treatment plans for patients by assessing their response to therapy in real time. Theranostics are particularly promising for the treatment of certain forms of cancer, such as prostate cancer and neuroendocrine tumors. With new radiotracers and imaging agents, molecular imaging is becoming more specific and sensitive, enabling earlier and more accurate disease detection. In addition, hybrid imaging, such as PET-CT and PET-MRI, combines two or more imaging modalities to provide complementary information and improve diagnostic accuracy. These approaches combine functional and anatomical information, enabling a comprehensive overview of disease processes. Therefore, such developments and approaches are expected to drive the market growth in the future.
Radiopharmaceuticals Market: Tracer Type Overview
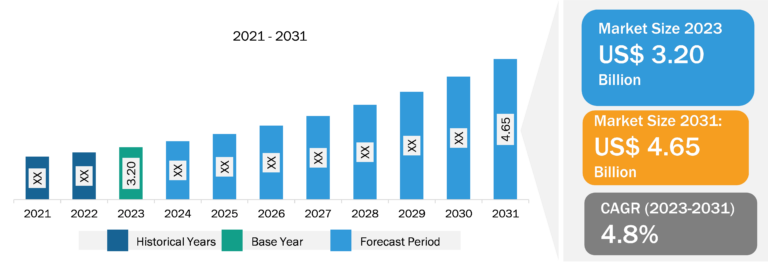
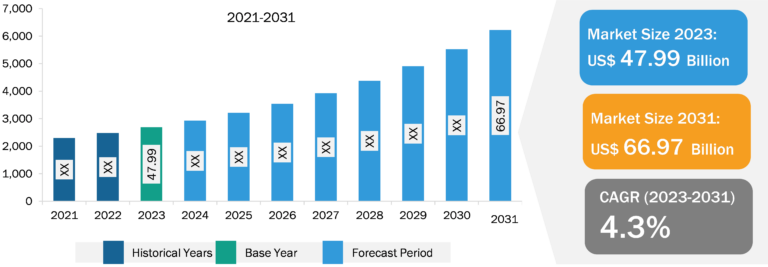
The radiopharmaceuticals market, based on tracer type, is segmented into Tc-99m, F-18, Ga-68, TL-201, I-131, Fe-59, Lu-171, RB-82 and N-13, Cr-51 and P-32, Sc-46, Sg-269 and Hs-269, and others. The Tc-99m segment held the largest radiopharmaceuticals market share in 2023 and is anticipated to register the highest CAGR during 2023–2031.
Technetium-99m is used in many medical diagnostic procedures, making it the most used medical radioisotope. Technetium-99m is utilized in morphological and dynamic imaging of various organs in the body to diagnose several diseases, including certain types of cancers. As per the National Center for Biotechnology Information, the US is the largest consumer of Tc-99m, carrying out more than 50% of all procedures that utilize Tc-99m radiopharmaceutical worldwide. Myocardial perfusion imaging for coronary artery disease drives the demand for Tc-99m owing to the high incidence of coronary artery disease in the US. A second major application is whole-body imaging for the detection of bone metastases. For instance, technetium-99m-MDP (i.e., methylene diphosphonate) is extensively used to detect bone metastasis. Other applications include sentinel node imaging for breast cancer or melanoma, thyroid, lung, and renal imaging before surgery.
Radiopharmaceuticals Market: Geographic Overview
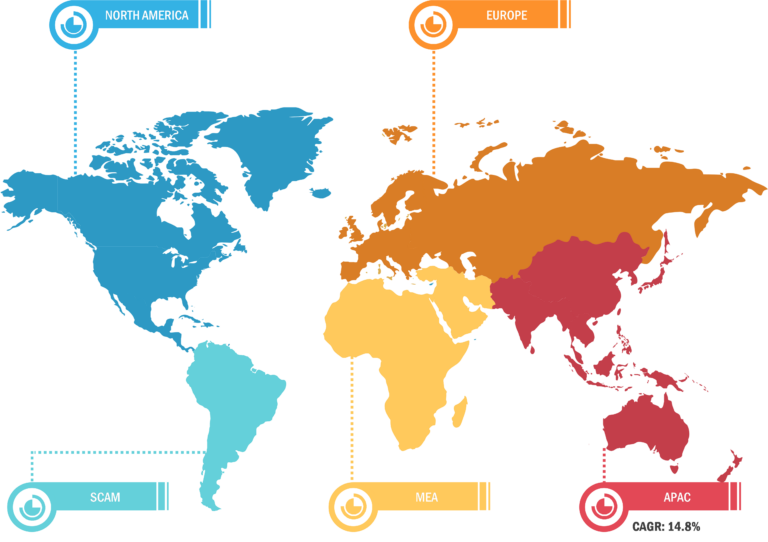
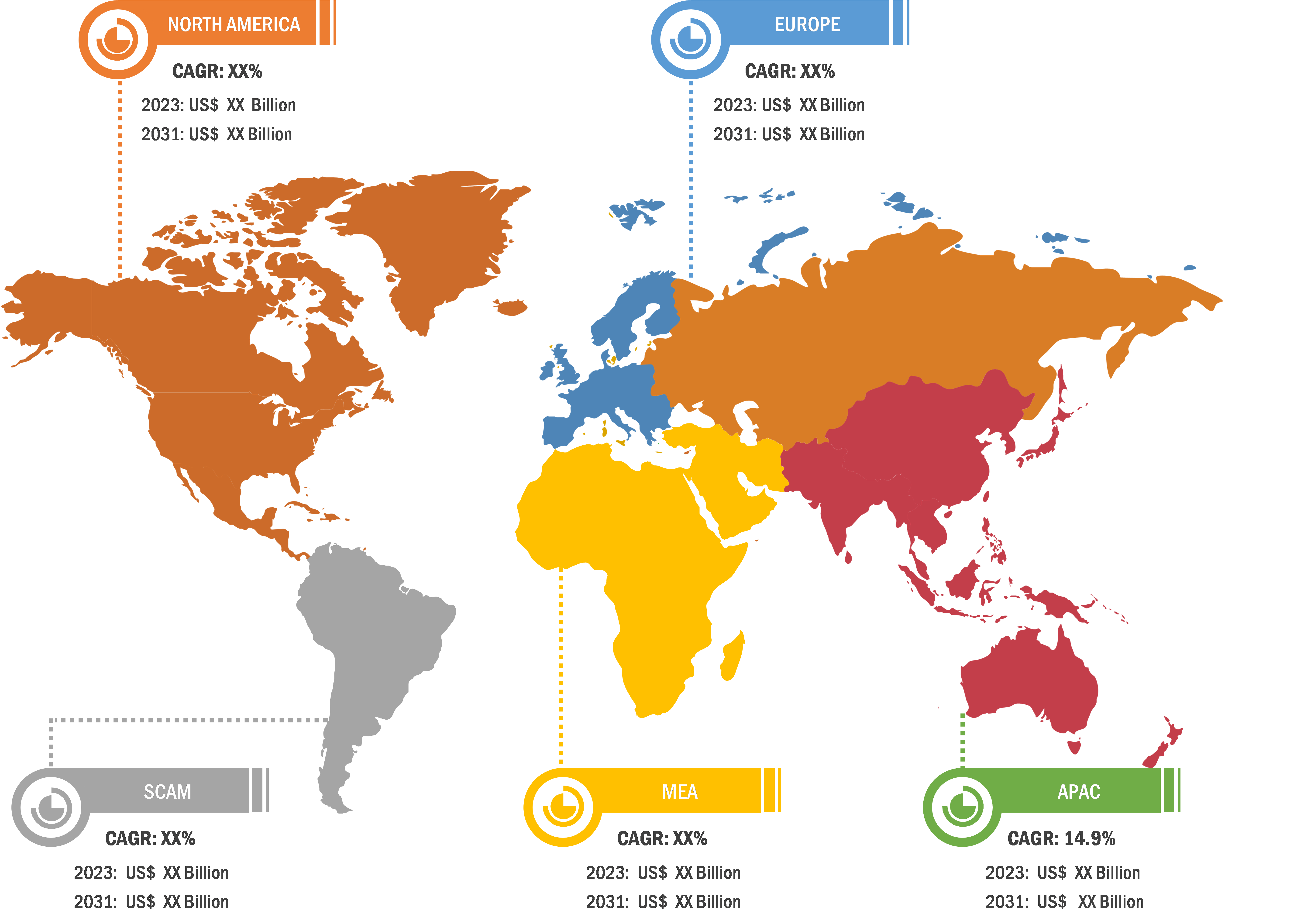
In terms of geography, the radiopharmaceuticals market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2023, North America accounted for the largest share of the radiopharmaceuticals market. The increasing incidences of chronic diseases, supportive government plans, growing strategic initiatives by the market players, and developments of innovative radiotherapies in the region determine market growth in the region. Asia Pacific is expected to register the highest CAGR during 2023–2031. China held the largest market share in 2023, and India is expected to show a significant growth rate in the market in Asia Pacific. After decades of development and rigorous efforts, a relatively mature radiopharmaceutical production and management system with high-quality facilities has been established in China. The National Medical Products Administration (NMPA) has successively issued related documents such as Guidelines for Technetium [99mTc] Radiopharmaceutical Quality Control and Guidelines for Positron Radiopharmaceutical Quality Control, as radiopharmaceuticals have a short physical half-life. Since 2020, the National Medical Products Administration (NMPA) has consecutively issued the technical guidelines for clinical evaluation of diagnostic radiopharmaceuticals and the technical guidelines for non-clinical research of diagnostic radiopharmaceuticals to promote and standardize the research, development, and clinical research of diagnostic radiopharmaceuticals in China. In August 2022, IBA (i.e., Ion Beam Applications S.A.), the world leader in particle accelerator technology and radiopharmaceutical manufacturing solutions, announced that it had signed a cooperation agreement with Chengdu New Radiomedicine Technology Co., Ltd (CNRT) for installing Cyclone IKON in Chengdu, Sichuan Province, China. The integration of diagnostics and therapy in nuclear medicine is expected to be further promoted in China and be more widely used in clinical practice. It is expected to become a routine diagnostic and treatment method for many diseases. Further, the growing number of market players focusing on countries in Asia Pacific for their geographic expansion and other growth strategies, the rising count of research centers, and a notable increase in government funding fuel the radiopharmaceuticals market growth in Asia Pacific.
Industry Developments and Future Opportunities:
As per company press releases, a few initiatives taken by leading players operating in the radiopharmaceuticals market are listed below:
- Curium announced that the marketing authorization application for PYLCLARI was submitted by an exclusive Swiss distributor, b.e.imaging AG, on January 31, 2024, to the Swiss Agency for Therapeutic Products (Swissmedic) and has been accepted for evaluation. PYLCLARI is indicated for the detection of prostate-specific membrane antigen (PSMA) positive lesions with positron emission tomography (PET) in adults with prostate cancer. (Curium, Press Release, January 2024)
- IBA Radiopharma Solutions announced that the Cyclone 70 system installed at the iThemba Laboratory for Accelerator-Based Sciences (iThemba LABS) in Cape Town, one of the National Research Foundation of South Africa research facilities, had been fully commissioned. This enabled iThemba LABS to start transferring the production of radioisotopes from their existing accelerator to the new Cyclone 70 system. (Source: IBA, Press Release, January 2024)
- Blue Earth Diagnostics Ltd announced that it has signed an exclusive strategic agreement with Sinotau Pharmaceutical Group for the positron emission tomography (PET) imaging agent flotufolastat (18F) injection (formerly referred to as 18F-rhPSMA-7.3) in prostate cancer. (Source: Blue Earth Diagnostics Ltd, Press Release,October 2023)
- Curium announced that it has entered into an exclusive rights agreement with b.e. Imaging, for the distribution of PYLCLARI (Piflufolastat (18F) formerly known as (18F)-DCFPyL), indicated for the detection of prostate-specific membrane antigen (PSMA) positive lesions with positron emission tomography in adults with prostate cancer. Under the terms of the agreement, b.e. Imaging would be responsible for obtaining marketing authorization from Swiss competent authority Swissmedic and would have exclusive distribution rights for PYLCLARI across Switzerland. (Source: Curium, Press Release, September 2023)
- Telix Pharmaceuticals Limited has advanced a partnership with Invicro LLC to develop an artificial intelligence (AI) platform to accompany Telix’s PSMA-PET imaging agent, Illuccix® (kit for the preparation of gallium Ga 68 gozetotide) – known as TelixAI™. TelixAI™ seeks to increase the efficiency and reproducibility of clinicians’ imaging assessments using advanced analysis capabilities with an initial focus on prostate cancer. (Source: Telix Pharmaceuticals Limited, Press Release, June 2022)
Radiopharmaceuticals Market: Competitive Landscape and Key Developments
Medi-Radiopharma (MRP), Rotem Industries Ltd., ABX Advanced Biochemical Compounds GmbH, Invicro LLC, Cardinal Health, Newcastle University, Novartis, Curium, Blue Earth Diagnostics, General Electric Company, and IBA Radiopharma Solutions are among the leading companies operating in the radiopharmaceuticals market. These players focus on expanding and diversifying their market presence and acquiring a novel customer base, thereby exploiting attractive business opportunities prevailing in the radiopharmaceuticals market.