Epilepsy Market
Epilepsy is one of the most common chronic neurological disorders across the world. Associated with an abnormal electrical activity in the brain, this disorder affects over 1 million American women of childbearing age. The rising prevalence of epilepsy and increasing investments in the development of epilepsy therapies are noteworthy factors contributing to the expansion of the epilepsy market size. The administration of anti-epileptic drugs (AEDs) is usually referred to as the first-line treatment for epilepsy. Approximately 80% of the people suffering from epilepsy are from developing countries. In children as well as adults, the treatment of epilepsy is mostly based on the use of drugs (either in monotherapy or combined therapy), while the remaining treatment approaches, such as surgery, neuromodulation, and ketogenic diet, are used at a lesser frequency. On the other hand, the recall of products hinders the growth of the epilepsy market.
Increasing Investments in Development of Epilepsy Therapies Drives Epilepsy Market Growth
The healthcare sector is transforming with rapid technological developments and innovation, enhancing healthcare services and quality of care. The epilepsy market is characterized by the presence of several small and large companies, and organizations implementing various growth strategies. The availability of funding enables them to boost production levels, expand into new markets, invest in new/improved technologies, and allocate more resources to R&D. A few of the notable investments in the epilepsy market, contributing to the market progress, have been mentioned below.
• In May 2023, Cadence Neuroscience secured US$ 26 million in Series B financing, which was led by Angelini Lumira Biosciences Fund and other new investors, including LivaNova USA, F-Prime Capital, Spectrum Financial Services, and Mayo Clinic, along with the company’s lead Series A investor—JAZZ Venture Partners. Cadence is a clinical-stage company focused on developing an innovative neuromodulation therapy for treating pediatric and adult patients with focal drug-resistant epilepsy. The therapy employs chronic subthreshold cortical stimulation to modulate EEG biomarkers associated with epilepsy to lower or eliminate seizures.
• In May 2023, Japan’s JCR Pharmaceuticals secured US$ 505.5 million from Angelini, a family-owned Italian pharmaceutical group, for the development of a new anti-epileptic treatment capable of penetrating the blood-brain barrier.
• In March 2023, the Swedish government decided to commission Vinnova, Sweden’s innovation agency, a total of US$ 7.7 million (80 million SEK) to establish a national innovation cluster for commercialization, skills development, and production capacity build-up for cell therapies as well as other advanced treatments such as gene therapy.
• In November 2022, scientists at Aston University in the UK, working on a project to develop new drug treatments to prevent the beginning of childhood epilepsy, were awarded US$ 2.4 million (£2 million) to discover the disease mechanism in the brain and the ways of epilepsy prevention. This three-year project funded by the Medical Research Council is a collaboration led by researchers in the College of Health and Life Sciences at Aston University, combined with Bristol University and Jazz Pharmaceuticals.
• In July 2022, Cerebral Therapeutics secured US$ 40 million in the Series C round funding to get its implanted infusion epilepsy treatment ready for phase 3 development. The funding round was led by Lynx1 Capital Management, among other investors, including RA Capital Management and Perceptive Advisors. Cerebral Therapeutics intends to deliver the specified levels of valproate to the central nervous system without causing weight gain, which was witnessed in subjects of oral formulation studies.
• In November 2021, Neurava, a startup located in Indiana, received over US$ 0.65 million in seed funding led by Elevate Ventures with participation from Purdue Foundry, UCB Biopharma, First Leaf Capital, iO Life Ventures, and angel investors. The company is working on the development of a wearable device to monitor and alert users about the approaching risk of sudden, unexpected death caused by epilepsy.
• In September 2021, the University of California San Diego won a US$ 12.25 million grant from the National Institutes of Health for projects focused on developing and enhancing brain-sensing and brain-stimulating platform technologies to aid the treatment of drug-resistant epilepsy.
Therefore, increasing investments in the development of advanced treatments propels the epilepsy market growth.
Epilepsy Market: Segmental Overview
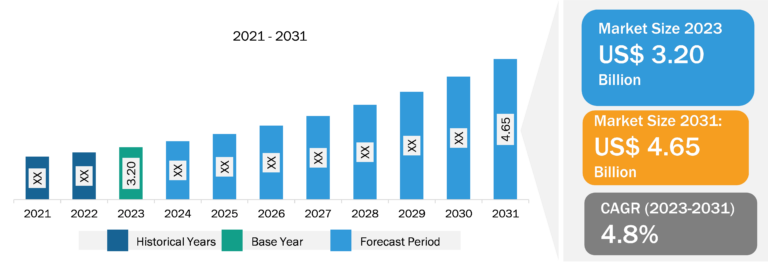
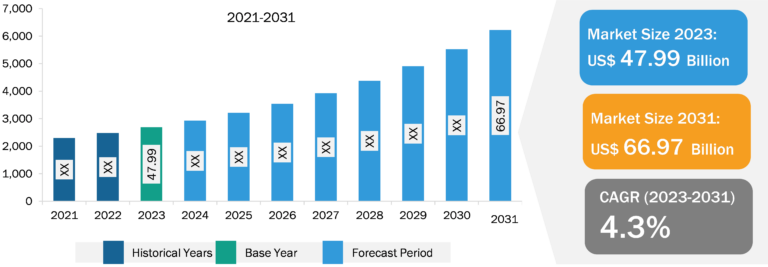
The epilepsy market is segmented on the basis of type, route of administration, treatment type, age group, and distribution channel. Based on treatment type, the market is segmented into first-generation drugs, second-generation drugs, and third-generation drugs. The third-generation drugs segment held a significant epilepsy market share in 2022. The segment is estimated to register the highest CAGR during 2022–2030. Third-generation AEDs, identified as advanced or next-generation AEDs, mainly include eslicarbazepine acetate, lacosamide, and cannabidiol. The drugs are claimed to be superior to the first- and second-generation AEDs in terms of safety and efficacy. The majority of new AEDs are used to control focal seizures, as well as specific epileptic syndromes (Lennox–Gastaut syndrome and Dravet syndrome) and tuberous sclerosis. Most of the third-generation AEDs exhibit fewer adverse effects, resulting in increased patient treatment adherence. These drugs are also effective, safe, and generally well-tolerated in children. Third-generation drugs are gaining importance but are not prescribed as frequently as second-generation anti-epileptic drugs.
Epilepsy Market: Competitive Landscape and Key Developments
Abbott Laboratories, Pfizer Inc, Eisai Co Ltd, UCB SA, CombiGene AB, Livanova PLC, Novartis AG, Medtronic PLC, GSK PLC, and H. Lundbeck AS are a few of the key companies operating in the market. These companies focus on product innovation strategies to meet evolving customer demands, along with maintaining their brand name in the epilepsy market.
A few of the recent developments in the global epilepsy market are mentioned below:
- In October 2022, Biohaven Pharmaceuticals declared its intention to develop treatments for neurological and neuropsychiatric conditions, after it was acquired by Pfizer Inc. The company is concentrating on BHV-7000, an investigational therapy for epilepsy that targets the Kv7 ion pathway.
- In March 2022, UCB SA acquired Zogenix Inc. to add more value to treatments designed for those suffering from severe forms of epilepsy. Together, they have plans to commercialize FINTEPLA to more individuals with uncommon epilepsies across the world, especially after it received regulatory approval for new indications such as Dravet syndrome in Japan and Lennox-Gastaut syndrome in the US and, more recently, in the EU.