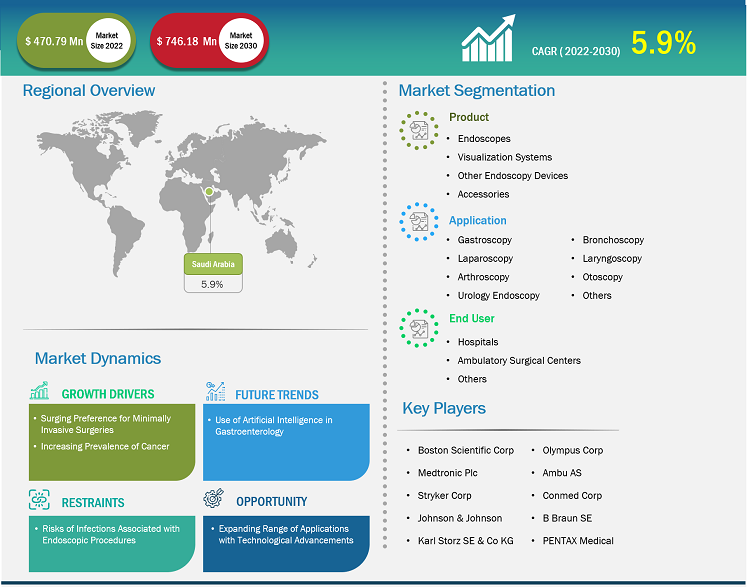
Saudi Arabia Endoscopy Devices Market
Increasing Prevalence of Cancer Drives Saudi Arabia Endoscopy Devices Market Growth
An increase in the prevalence of cancer drives the demand for endoscopy devices. These devices are used as vital tools for diagnosis (in biopsies), treatment, and monitoring during cancer treatments. Endoscopy procedures enable the early detection and diagnosis of various types of cancers, including esophageal, pancreatic, colorectal, and stomach cancer. Through the use of endoscopes and imaging technologies, healthcare providers can visualize the internal structures of organs to identify abnormalities, lesions, and early-stage tumors, thereby facilitating timely intervention and improved treatment outcomes.
According to Globocan 2020, the number of cancer cases reported in the world was 19.29 million in 2020, which is expected to reach 30.23 million cases by 2040. The age-standardized prevalence rate of all cancer cases globally was 190 cases per 100,000, and the age-standardized prevalence rate of all cancer cases in Saudi Arabia was 95.2 cases per 100,000. As per the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO), Saudi Arabia records a high prevalence of colorectum cancer, breast cancer, thyroid cancer, non-Hodgkin lymphoma, leukemia, lung cancer, and liver cancer, among others. As per estimates by IARC, the number of all cancer cases in Saudi Arabia is expected to grow from 27,885 in 2020 to 60,429 by 2040. As the burden of cancer continues to rise in Saudi Arabia and the world, the adoption of endoscopy in cancer diagnostics and care becomes increasingly vital. Thus, the ongoing developments in endoscopic techniques are aimed at addressing the complexities that influence cancer intervention. Thus, the rising prevalence of cancer fuels the adoption of endoscopy devices globally and in Saudi Arabia.

Saudi Arabia Endoscopy Devices Market: Segmental Overview
The Saudi Arabia endoscopy devices market, by product, is segmented into endoscopes, visualization systems, accessories, and other endoscopy devices. The endoscopes segment held the largest share of the market in 2022, and the visualization systems segment is anticipated to register the highest CAGR in the market during 2022–2030. An endoscope is used in minimally invasive surgeries and can be inserted into the openings of the body. The device is commonly used to visually examine internal organs and accordingly diagnose medical conditions. However, some of these devices are also used for small surgical tasks due to their fine, flexible design, which helps reduce cost, time, and physical trauma that are associated with standard surgical procedures. Most of the endoscopes are similar in construction. The endoscopes are named as per the location of their use. For example, a nephroscope is used for the endoscopy of the kidney, an arthroscopy is used for joints, and a laparoscope for the endoscopy of the abdomen or pelvis. There are various types of endoscopes based on their structure, such as rigid, flexible, capsule, and robot-assisted endoscopes.
Based on application, the Saudi Arabia endoscopy devices market is segmented into gastroscopy, laparoscopy, arthroscopy, urology endoscopy, bronchoscopy, laryngoscopy, otoscopy, and others. The gastroscopy segment held the largest share of the market in 2022. However, the laparoscopy segment is expected to register the highest CAGR in the market from 2022 to 2030. Gastroscopy, also known as upper endoscopy, involves examining the upper gastrointestinal tract—the stomach, esophagus, and duodenum (the beginning part of the small intestine). It is used to diagnose conditions such as ulcers, tumors, inflammation, and gastroesophageal reflux disease (GERD). Laparoscopy, commonly referred to as minimally invasive surgery, involves using a laparoscope to visualize the abdominal or pelvic cavities for diagnostic or surgical purposes. This approach offers reduced incision size, faster recovery, and lower complication risks compared to traditional open surgeries.
Based on end user, the Saudi Arabia endoscopy devices market is segmented into hospitals, ambulatory surgical centers, and others. The hospitals segment held the largest share of the market in 2022, and the same segment is expected to register the highest CAGR in the market from 2022 to 2030. Hospitals encompass a wide spectrum of medical facilities, ranging from community hospitals to large academic medical centers. They serve as major end users of endoscopy devices. The demand for endoscopy devices within hospital settings is driven by factors such as comprehensive care delivery, inpatient and outpatient services, and advanced procedures and interventions.
Saudi Arabia Endoscopy Devices Market: Competitive Landscape and Key Developments
Boston Scientific Corp, Medtronic Plc, Stryker Corp, Johnson & Johnson, Karl Storz SE & Co KG, Olympus Corp, Ambu AS, Conmed Corp, B Braun SE, and PENTAX Medical are a few key companies operating in the endoscopy devices market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the endoscopy devices market.
A few of the recent developments in the endoscopy devices market are mentioned below:
- In October 2023, Olympus Corp announced the launch of the next-generation EVIS X1 endoscopy system in the market. The company had plans to showcase and demonstrate the product during October 22–24, 2023, at the yearly meeting of the American College of Gastroenterology (ACG) in Vancouver, Canada. With the amazing new tools available for GI tract visualization, doctors will be able to make better observations and assist their patients more effectively.
- In October 2023, Olympus announced the release of its EU-ME3 endoscopic ultrasound processing platform. The EU-ME3 meets medical practitioners’ demands for high-quality images during endoscopic ultrasounds. During this fiscal year, the company intends to launch the system in Europe, the Middle East, Africa, sections of Asia, and Oceania.
- In September 2023, Ambu expanded its gastroenterology portfolio with the announcement of the Ambu aScope Gastro Large and Ambu aBox 2, two new larger gastroscopy solutions that will be available in Europe. In addition to being the first gastroscope in the world with a 4.2 mm operating channel, which enables gastroenterologists to achieve strong suction performance during procedures in ICUs and endoscopy units, the Ambu aScope Gastro Large is the first endoscope ever manufactured of bioplastic materials.