Radiopharmaceutical Therapeutic Market
Radiopharmaceuticals precisely target and deliver high doses of radiation directly to the site of the disease. The growing popularity of targeted therapies and the increasing prevalence of cancer are noteworthy factors contributing to the growing radiopharmaceutical therapeutic market size in the world. Moreover, the growing number of market players focusing on research and development activities in the field of imaging technology and the increasing number of technologically advanced product launches are likely to propel the growth of the market. In addition, high investments in R&D and support from the government for the use of medical isotopes are bolstering the market growth in the US. However, the short shelf-life of radiopharmaceuticals hinders the growth of radiopharmaceutical therapeutic market.
Increasing Prevalence of Cancer Drives Radiopharmaceutical Therapeutic Market Growth
According to the study titled “Spotlight on Gene Therapy in China,” published in July 2020, ~4 million new cancer cases are recorded per year in the country. According to the World Cancer Research Fund International Data, ~1.02 million cases of cancer were diagnosed in 2020 in Japan. The age-standardized rate for all cancers was 282.9 per 100,000 in 2020. Additionally, as per the National Cancer Registry Program Report 2020, in India, ~0.67 million cancer cases were reported in males, and the number is anticipated to increase to ~0.76 million by 2025, whereas in females, ~0.71 million cancer cases were recorded in 2020, and the number is predicted to reach ~0.81 million by 2025.
As per the American Cancer Society’s 2023 report, ~43,720 new thyroid cancer cases (12,540 in males and 31,180 in females) were recorded in 2023. In addition, 2,120 deaths occurred from thyroid cancer (970 in males and 1,150 in females) in the same year. As per the American Society of Clinical Oncology, prostate cancer is a common type of cancer in males. In the US, ~34,500 people died due to this disease in 2020. As per the American Cancer Society’s estimates for prostate cancer, ~299,010 new cases of prostate cancer will occur in the US in 2024. Older men are more likely to develop prostate cancer. About 6 out of 10 instances are seen among men aged 65 and above. Radiopharmaceutical therapy involves targeting cancer cells with a radioactive drug (radiopharmaceutical). Radiopharmaceutical therapy specifically targets cancer cells, reducing damage to healthy tissue. This type of therapy offers promise as a means for personalized cancer treatment since it can be modified to the molecular properties of a specific tumor. Radiopharmaceutical therapeutics are currently used to treat thyroid cancer, prostate cancer, and well-differentiated neuroendocrine tumors. For instance, I-131 is used in therapy for hyperthyroidism and thyroid cancer. Thus, the need for radiopharmaceutical therapeutics is increasing due to the rising prevalence of chronic diseases, thereby driving the radiopharmaceutical therapeutic market growth.
Radiopharmaceutical Therapeutic Market: Segmental Overview
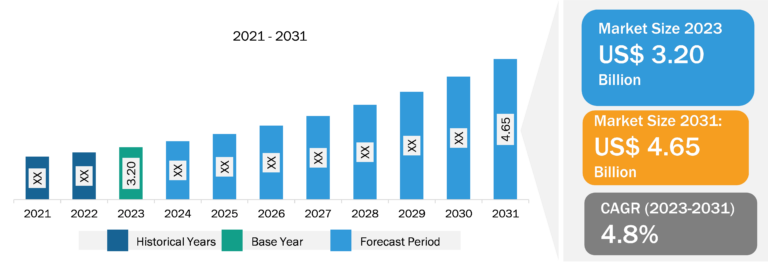
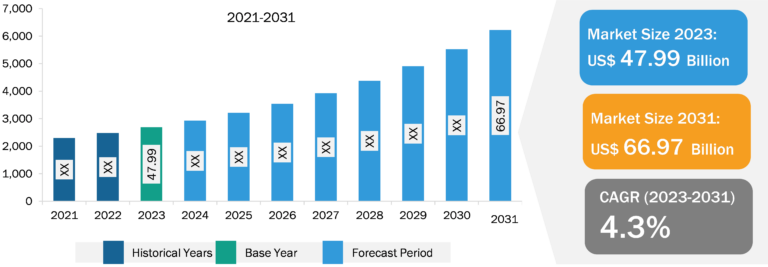
The radiopharmaceutical therapeutic market is segmented on the basis of product type, therapy type, application, and end user. Based on application, the market is segmented into oncology, neurology, cardiology, and others. The oncology segment held a significant radiopharmaceutical therapeutic market share in 2023 and is estimated to register the highest CAGR during 2023–2031. Numerous radiopharmaceuticals are routinely used in clinical practice, such as Gallium-68, and dozens are under preclinical development. Therapeutic radiopharmaceuticals for cancer treatment are primarily labeled with beta-emitting radionuclides. The radionuclides I-131 are frequently used for this purpose due to a greater success rate with the use of radiopharmaceutical therapeutics in prostate cancer. In March 2022, Novartis received the US Food and Drug Administration (FDA) approval for Pluvicto (lutetium Lu-177 vipivotide tetraxetan) for treating adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). It is the first FDA-approved targeted radioligand therapy that links a targeting compound (ligand) with a therapeutic radioisotope (a radioactive particle) for eligible patients with mCRPC.
Radiopharmaceutical Therapeutic Market: Competitive Landscape and Key Developments
Cardinal Health, Bayer, Novartis, Jubilant DraxImage Inc., Curium, Yantai Dongcheng Pharmaceutical Group, Lantheus, Q BioMed, Telix Pharmaceuticals, and Radiopharm Theranostics are a few of the key companies operating in the market. These companies focus on product innovation strategies to meet evolving customer demands, along with maintaining their brand name in the radiopharmaceutical therapeutic market.
As per company press releases, a few recent developments initiated in the global radiopharmaceutical therapeutic market are mentioned below:
- In October 2022, NuView Life Sciences, Inc. is a clinical-stage precision oncology company that focuses on advanced approaches, such as theranostics, to identify and treat cancer. The company leveraged its clinical-stage small molecule, NV-VPAC1, to develop its unique theranostic technology for precision diagnostics and targeted therapy. NV-VPAC1 binds directly to VPAC1 receptors located in high densities in breast cancer tumors. With FDA approval being sought for NV-VPAC1, there is the potential to attain tremendous benefit.
- In March 2022, Novartis received the US FDA approval for Pluvicto (lutetium Lu 177 vipivotide tetraxetan) for treating adult patients with PSMA-positive mCRPC that has spread to other parts of the body.
- In March 2022, Bracco Imaging launched a new subsidiary, Blue Earth Therapeutics, in order to advance the development of next-generation therapeutic radiopharmaceutical technology. Innovative rhPSMA Theranostic technology offers both diagnostic imaging and treatment for patients affected by prostate cancer.
- In June 2021, Bayer acquired Noria Therapeutics Inc. and PSMA Therapeutics Inc. to broaden Bayer’s existing oncology portfolio of targeted alpha therapies for cancer. Through the acquisition, Bayer obtained exclusive rights to a distinguished alpha radionuclide therapy based on actinium-225 and a small molecule targeting prostate-specific membrane antigens. This therapy could provide a distinct efficacy and safety profile and provide significant potential to tackle a high unmet medical need for men with prostate cancer.