Germany Procedure Trays Market
Escalating Demand for Customized Procedure Trays Would Lead to Future Trends in Market
Surgical departments face the issue when too many products are pulled for procedures that end up on the back table of the operating room (OR) suite—opened, unused, and thrown away. They need to bear the cost of discarded products and the labor cost of returning unopened items to inventory. This scenario leads to the wastage of both time and money. For instance, US$ 50 worth of waste through procedure trays per surgery can lead to a loss of US$ 250,000 annually for a surgery center that performs ~5,000 procedures yearly. Hospitals often have special requirements for treatment sets. Choices vary by doctor and even by theater staff. The growing variety of surgical procedures and preferences requires procuring a wide variety and quantity of supporting clinical products. Thus, surgical centers should optimize custom procedure trays to support quality outcomes while reducing waste, controlling costs, and improving workflows. Standardized and customized procedure trays can save time, eliminate waste, ensure the right components are at hand for each procedure, and streamline workflows. Surgery centers focus on including customized procedure trays in their supply chains to manage costs. Customized procedure trays are cheaper than traditional trays, and purchasing them in bulk may efficiently reduce procedures’ total procurement and operational cost. Thus, with the growing need for cost-effective products by hospitals and surgical centers, customized procedure trays are trending, boosting the market.
Germany Procedure Trays Market: Segmental Overview
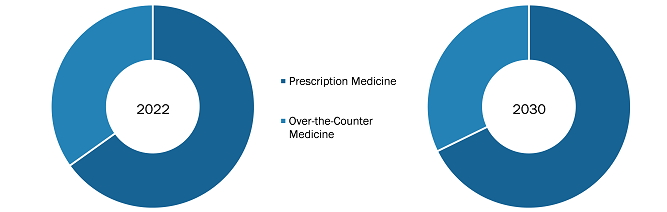
The Germany procedure trays market has been segmented based on application and end user. The market, by application, is segmented into operating room, angiography, ophthalmology, and others. The operating room segment held a larger market share in 2022 and will register with a higher CAGR during 2022–2030.
Based on end users, the Germany procedure trays market is bifurcated into ambulatory surgical centers, hospitals & clinics, and others. The hospitals & clinics segment held the largest share of the market in 2022 and is projected to register the highest CAGR during 2022–2030. Every healthcare facility includes the usage of tool trays. They are utilized in hospitals, laboratories, clinics, and emergency centers.
Germany Procedure Trays Market: Competitive Landscape and Key Developments
Biometrix, Medica Europe BV, 3M Co, BD, Owens & Minor Inc, Molnlycke Health Care AB, Nelipak Corporation, Teleflex Incorporated, Cardinal Health Inc, and ICU Medical Inc are among the leading companies in the Germany procedure trays market. These players focus on expanding and diversifying their market presence by acquiring a novel customer base, thereby tapping prevailing business opportunities in the Germany procedure trays market.
A few significant developments in the Germany procedure trays market are as follows.
- In May 2020, Nelipak Healthcare Packaging launched Nelipak Academy, a seven-part educational webinar series. The free series covers various topics, including automated handling trays, NeliSim, polymer basics and film properties, rigid barrier packaging design, lab testing services, and heat seal coating technology.
- In December 2021, Owens & Minor, Inc. acquired American Contract Systems (ACS), a Minnesota-based provider of kitting and sterilization services for Custom Procedure Tray (CPT) solutions. This acquisition will enhance combined abilities to serve customers with a stronger CPT offering.
- In October 2022, Pioneering med-tech company BD India partnered with the Government of India’s renowned research organization, Raja Ramanna Centre for Advanced Technology (RRCAT), to sterilize one of its medical devices, Venflon Pro, by electron beam (e-beam) technology, at RRCAT’s Indore facility. The e-beam facility is entirely designed and developed by scientists and engineers of RRCAT. It is the only facility approved by regulatory authorities for irradiation of class A and Class B medical devices in India and is also ISO–13485 certified.