Sialorrhea Treatment Market
Availability of Various Treatment Options for Sialorrhea Drives Sialorrhea Treatment Market Growth
Treatments for sialorrhea include behavioral and lifestyle counseling, medication, surgery, and radiation. Advice on posture, controlling swallowing, managing cough, dietary adjustments, eating and drinking procedures, and behavioral modification are a few examples of nonpharmacological therapies. Nevertheless, these conservative measures might not work for individuals affected by neurological problems that worsen over time. Many drugs that have been used to treat sialorrhea also have anticholinergic properties that have been utilized to control hypersalivation. Amitriptyline, procyclidine, and rihexyphenidyl are a few of these. The majority of medications have co-morbidities such as discomfort or trouble sleeping in addition to hypersalivation, which makes them potentially helpful in some situations. Therefore, the availability of various treatments for sialorrhea boosts the sialorrhea treatment market growth.
Diagnosis and treatment of chronic sialorrhea are delayed due to ignorance about the condition by patients and healthcare professionals. This factor hinders the market expansion for chronic sialorrhea treatments. Furthermore, it is anticipated that healthcare professionals—including specialists and primary care doctors—will know very little about persistent sialorrhea as a separate illness.
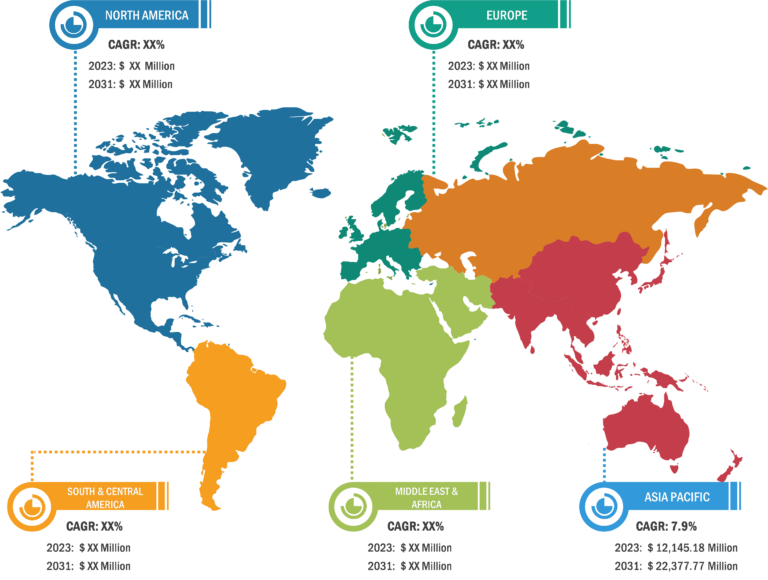
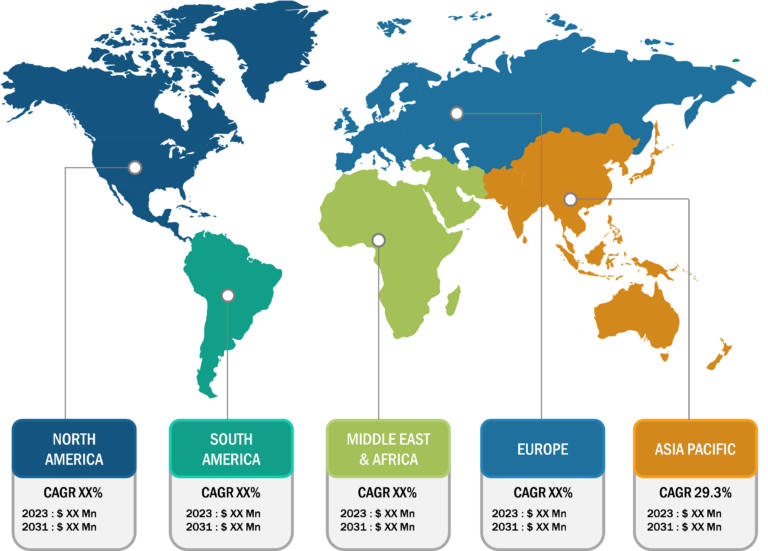
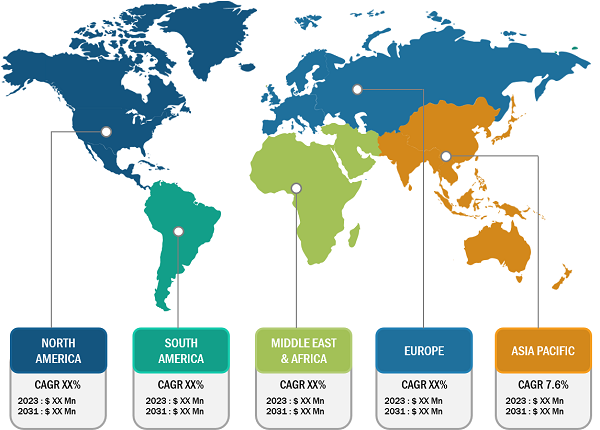
North America holds the largest share of the global sialorrhea treatment market. The US, Canada, and Mexico are major contributors to the regional market growth. However, Asia Pacific is anticipated to register the highest CAGR during the forecast period. The growth of the sialorrhea treatment market in North America is driven by increasing awareness of sialorrhea, better access to medical facilities, presence of major drug manufacturers, and high volume of screening procedures in the region. In North America, the US accounts for the largest market share. Patient suffering from Parkinson’s disease frequently exhibits drooling, which can be brought on by excessive salivation, difficulty swallowing, or an inability to hold saliva in the mouth. According to the Parkinson’s Foundation, in the US, around one million individuals suffer from Parkinson’s disease (PD). By 2030, this figure is anticipated to increase to 1.2 million. After Alzheimer’s disease, Parkinson’s is the second most prevalent neurological illness. Annually, about 90,000 US citizen receive a PD diagnosis.
Over 10 million people globally are affected by Parkinson’s disease. Parkinson’s disease is more common as people age, and only 4% of cases are identified before the age of 50. Parkinson’s disease is 1.5 times more common in men than in women.
Asia Pacific is expected to record the highest CAGR in the sialorrhea treatment market during 2022–2030. Growing government initiatives to increase awareness regarding this condition, increasing research and development activities in the region, growing market players’ shifts toward providing better treatment options are among the prominent factors propelling the sialorrhea treatment market growth..
Sialorrhea Treatment Market: Segmental Overview
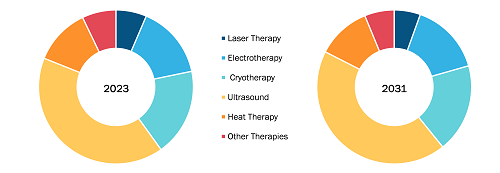
Based on medical therapy, the sialorrhea treatment market is segmented into pharmacologic therapy, radiotherapy, and others. In 2022, the pharmacologic therapy segment held the largest market share. Also, the segment is expected to record the highest CAGR during 2022–2030. Vaccines provide active acquired immunity against particular infectious diseases. In multiple independent clinical trials, pharmacological treatments such as benztropine, glycopyrrolate, scopolamine, and botulinum toxins have been demonstrated to be effective in managing sialorrhea. Medications are delivered orally or parenterally. Botulinum toxin is injected into the salivary gland guided by anatomical external palpation or ultrasonography.
The sialorrhea treatment market, by end user, is segmented into hospitals, specialty clinics, homecare settings, and others. The hospitals segment held the largest market share in 2022 and is anticipated to record the highest CAGR during 2022–2030. The treatment for sialorrhea is done in hospitals through appropriate treatment and motor therapy, with the help of highly experienced interdisciplinary medical specialists, including physiotherapists and neurology specialists.
Sialorrhea Treatment Market: Competitive Landscape and Key Developments
Pfizer Inc, F. Hoffmann-La Roche Ltd, Mylan N.V., Fresenius Kabi AG, Hikma Pharmaceuticals PLC, Novartis AG, Teva Pharmaceutical Industries Ltd., Bristol Myers Squibb Company, GSK Plc., Bayer AG, and Sun Pharmaceutical Industries Ltd are among the leading companies operating in the global sialorrhea treatment market. These players focus on expanding their business, diversifying their market presence, and acquiring a novel customer base, thereby tapping prevailing business opportunities.
- In February 2023, Proveca Pharma Ltd, a pharmaceutical company that specializes in medicines for children has provided Sialanar (glycopyrronium bromide) to children with chronic neurological disorders who suffer from severe sialorrhea in France.
- In September 15, 2016, Sialanar (320 mcg/ml glycopyrronium, oral solution) received a European Paediatric Use Marketing Authorisation (PUMA) for the symptomatic treatment of severe sialorrhea (chronic pathological drooling) in children and adolescents aged 3 years and above with chronic neurological disorders.
- In March 2022, ExCEEd Orphan announced its partnership with Proveca Pharma Ltd to distribute and commercialize Sialanar, a medication to treat severe sialorrhea in children and adolescents. The rights are for eight Central and Eastern Europe (CEE) countries, including Bulgaria, Croatia, the Czech Republic, Hungary, Poland, Romania, Slovakia, and Slovenia.
- In August 2021, Merz Therapeutics, a business of the Merz Group and a leader in the field of neurotoxins, was granted the use of XEOMIN for the symptomatic treatment of chronic sialorrhea in children and adolescents aged 2 to 17 years and weighing ≥ 12 kg due to neurodevelopmental disorders/neurological on EU level. The US Food and Drug Administration approved it in December 2020, and the Russian Federal Service for Surveillance in Healthcare August 2021.