Deep Brain Stimulation Market
Deep brain stimulator (DBS) is a device comprising a brain-oriented pacemaker (an implantable pulse generator) that sends electrical stimulation via surgically implanted electrodes to specific targets in the brain. DBS is generally used to treat movement disorders and neuropsychiatric disorders. The rising prevalence of neurological disorders and increasing demand for minimally invasive surgery are driving the deep brain stimulation market size. However, side effects associated with deep brain stimulation hinder the deep brain stimulation market growth.
Increasing Demand for Minimally Invasive Surgery Drives Deep Brain Stimulation Market Growth
Deep brain stimulation is a minimally invasive targeted surgery used to treat movement disorders, including dystonia, Parkinson’s disease, and essential tremor. Minimally invasive surgeries (MIS) offer several advantages over traditional surgical techniques. MIS minimizes trauma to the body, reduces postoperative pain, increases recovery speed, and improves outcomes. Robotic procedures represent the latest evolution in minimally invasive procedures, providing surgeons with precision equipment that uses the same small incisions used in traditional laparoscopy. MIS leads to less blood loss, less analgesic use, and shorter postoperative hospital stay than open surgery. In addition, minimally invasive surgery indicates a higher accuracy rate than traditional surgical methods. Also, these procedures require tiny incisions large enough to insert thin tubes and cameras. These benefits of MIS are increasing inpatients and have encouraged older patients who face high operative morbidity and mortality to seek surgery. Thus, the rising demand for minimally invasive surgeries bolsters the growth of the deep brain stimulation (DBS) market.

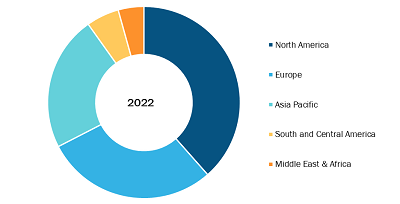
North America accounted for the largest share of the global deep brain stimulation market in 2022. In the region, the US held the largest deep brain stimulation market share and is anticipated to register the highest CAGR during the forecast period. The market in the country is growing owing to the rising prevalence of neurological diseases such as Parkinson’s disease (PD), increasing awareness about neurological disorders, and growing investments in developing transcranial stimulators. According to a study titled “2022 Alzheimer’s disease facts and figures” published in the Alzheimer’s Association, ~6.5 million Americans aged 65 and above suffered from Alzheimer’s disease in 2022, and the number is projected to rise to 13.8 million by 2060. In addition, according to the Parkinson’s Foundation, ~1 million people in the US are suffering from Parkinson’s disease, which is expected to reach 1.2 million by 2030. Moreover, in January 2020, Abbott’s Infinity DBS System received US Food and Drug Administration (FDA) approval for treating Parkinson’s disease. Thus, the above-mentioned factors propel the demand for DBS solutions, which fuels the deep brain stimulation systems market.
Deep Brain Stimulation Market: Competitive Landscape and Key Developments
Abbott Laboratories, Aleva Neurotherapeutics SA, Medtronic Plc, Beijing PINS Medical Co Ltd, Boston Scientific Corp, Newronika SpA, and SceneRay Co Ltd are a few key companies operating in the deep brain stimulation market. Deep brain stimulation companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the deep brain stimulation market.
A few of the recent developments in the global deep brain stimulation market are mentioned below:
- In September 2022, Aleva Neurotherapeutics received CE-mark approval for its magnetic resonance imaging (MRI) labeling for the directSTIM DBS system. The directSTIM system, featuring a fully directional lead, is designed to treat the symptoms of Parkinson’s disease by delivering precisely targeted electrical stimulation to the brain, providing optimal symptom relief and better control of unwanted side effects.
- In January 2021, Boston Scientific Corp received the US Food and Drug Administration (FDA) approval for its fourth-generation Vercise Genus DBS System. The portfolio, approved for conditional use in an MRI environment, consists of Bluetooth-enabled, rechargeable, and non-rechargeable, implantable pulse generators (IPGs) that power Cartesia Directional Leads, designed to provide optimal symptom relief.