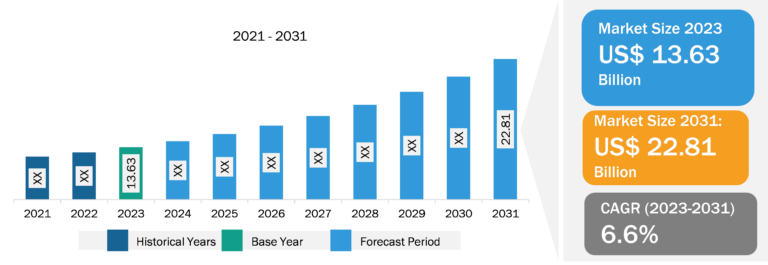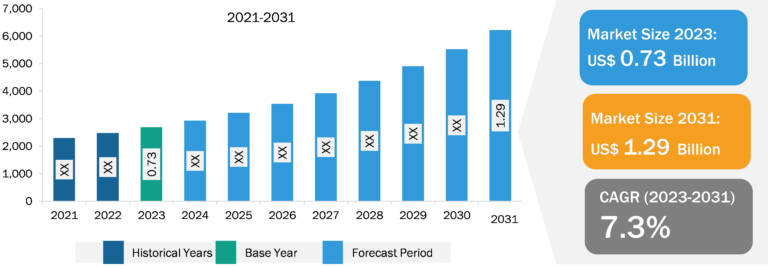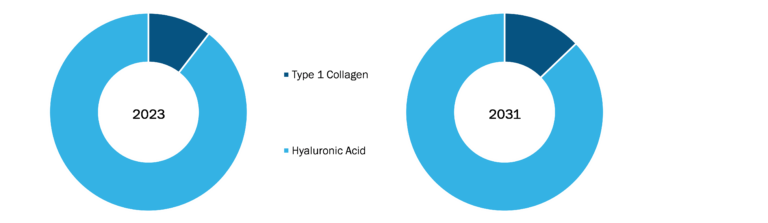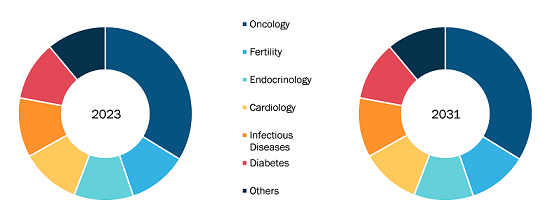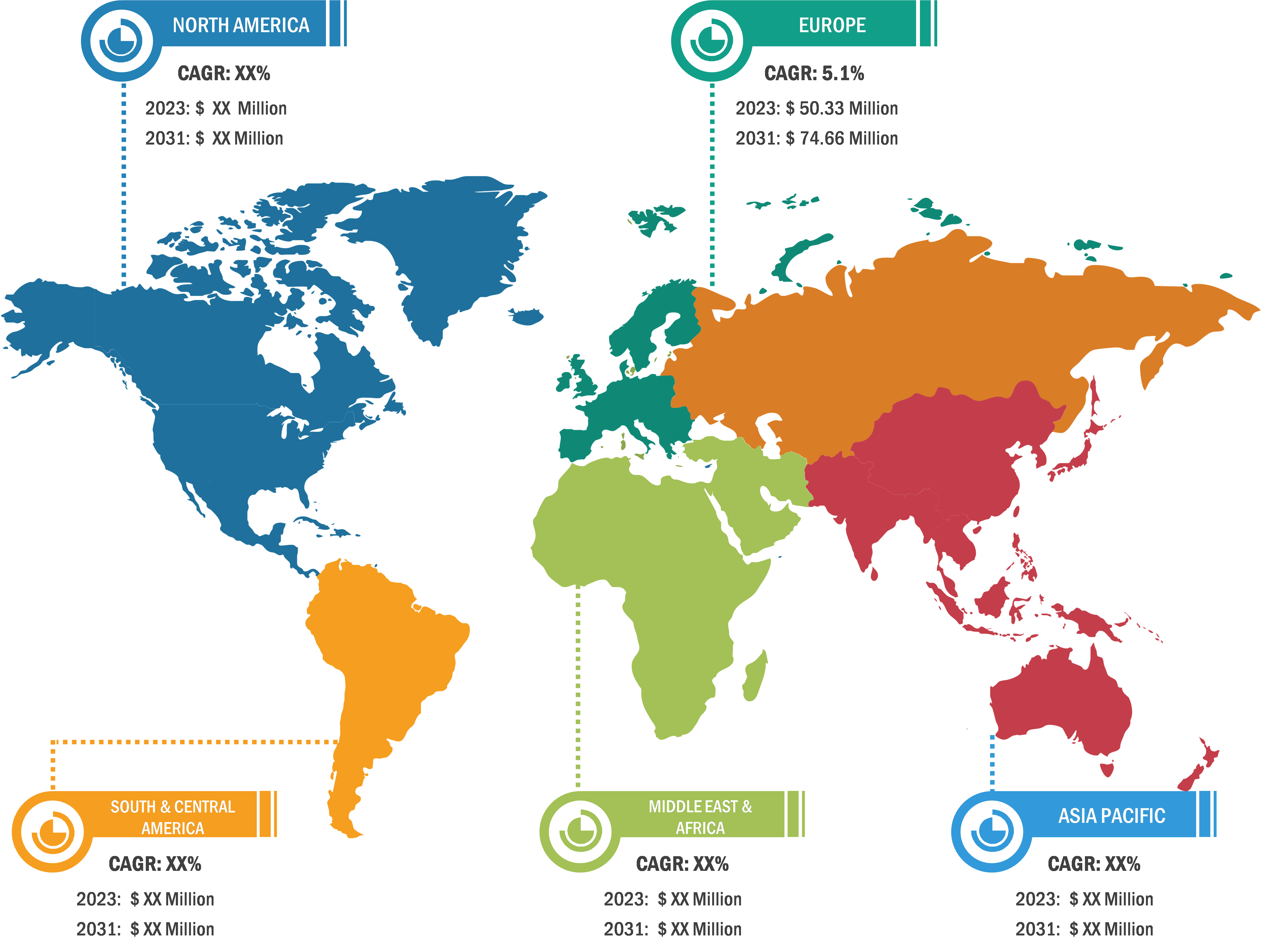
Balloon Aortic Valvuloplasty Market
Aortic valvuloplasty is a procedure for repairing the aortic valve of the heart. It is utilized as a bridge to aortic valve replacement surgery or to enhance the quality of life for patients who are not eligible for major heart surgery. Balloon valvuloplasty is the minimally invasive treatment used to open constricted heart valves. A catheter with a tiny, deflated balloon attached to its tip is used in the process. The balloon is inflated to release the impediment and open the valve. Balloon valvuloplasty cures cardiovascular device symptoms and enhances blood flow. Many patients who require isolated valve surgery can be treated robotically or minimally invasively, which speeds up recovery and reduces complications. Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure in which a narrowed aortic valve is replaced, and this procedure is used for the treatment of aortic stenosis. As per the data published in the Journal of the European Society of Cardiology in February 2020, aortic stenosis is the second most common valvular lesion reported in the US. It is present in approximately 5% of the population above the age of 65. Additionally, the increasing demand and awareness regarding minimally invasive treatment approaches for the treatment and management of cardiovascular diseases positively impact the balloon aortic valvuloplasty market during the forecast period. The increasing demand for minimally invasive procedures and the rising prevalence of aortic valve stenosis are noteworthy factors contributing to the expansion of the balloon aortic valvuloplasty market size. On the other hand, the high cost of procedures hinders market growth. Moreover, improvements in TAVI combined with research and development are expected to bring new balloon aortic valvuloplasty market trends in the coming years.
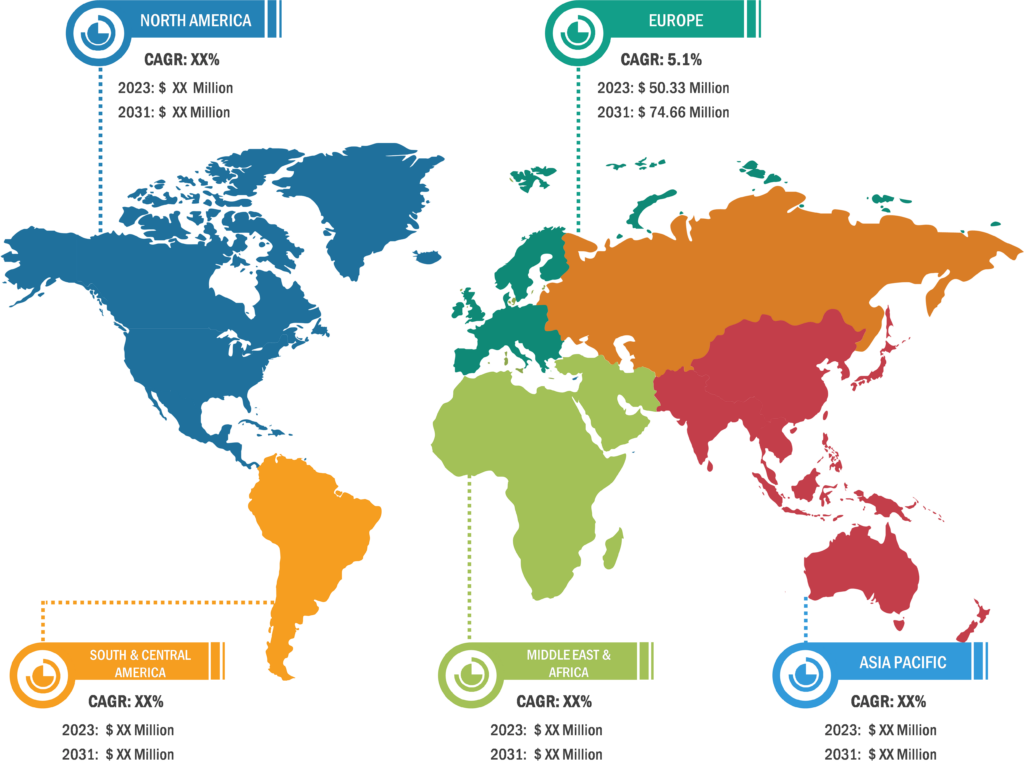
Rising Prevalence of Aortic Valve Stenosis Drives Balloon Aortic Valvuloplasty Market Growth
Heart valve disease (HVD) is a cardiac defect increasingly linked to functional decline, especially in aging individuals. HVD can cause stenosis, regurgitation, or both. As the incidence of HVD prominently increases with aging, it is estimated to affect millions globally in the upcoming years. According to the Institute of Health Economics 2022, aortic stenosis and mitral regurgitation are the two common HVDs. As per the estimates, 1.5 million people aged 65 and above are expected to suffer from HVD by 2040 in Canada.
The narrowing of the aorta can make it difficult for blood to flow from the heart to various parts of the body, resulting in a condition called aortic stenosis. As the heart is under considerable pressure while pumping blood through the constricted aorta, it leads to symptoms such as shortness of breath, chest pain, and fatigue. The high-risk patient populations are inoperable and cannot undergo surgical aortic valve replacement procedures. The risk of developing aortic valve stenosis rises with age. As per the study titled “Epidemiology of aortic valve stenosis and aortic valve incompetence (AI),” published in February 2020, in Europe and Taiwan, aortic stenosis had a prevalence rate of 12.4% and severe aortic stenosis reported a prevalence rate of 3.4% in patients aged 75 years and over. Aortic stenosis prevalence exponentially increases with age, displaying 0.2% in people aged 50–59 years, 1.3% in the 60–69 year group, 3.9% in the 70–79 year group, and 9.8% in people aged 80–89 years. The high prevalence of aortic valve stenosis is surging the demand for BAV devices. BAV is less invasive than conventional open-heart surgery, making it a more attractive option for patients.
According to the research study titled “Uncovering the treatable burden of severe aortic stenosis in the UK,” published in 2021 in the open-access journal Open Heart, ~300,000 people have aortic valve stenosis—a potentially deadly heart condition—in the UK. Of the people with aortic stenosis, ~199,000 (68%) reported to have severe aortic stenosis disease. Additionally, the National Echo Database of Australia data 2021 reveals that 97,379 Australians suffered from severe aortic stenosis. The number of moderate to severe aortic stenosis cases in Australia is estimated to grow to 200,000 patients in 2031 and 266,000 by 2051.
Similarly, India is also overburdened with aortic stenosis. The number of fatalities due to calcific aortic valve disease has increased significantly over the past decade in the country. Transcatheter aortic valve implantation (TAVI), also called transcatheter aortic valve replacement (TAVR), has become a well-established therapeutic option for severe aortic stenosis. BAV is a crucial procedural step during TAVI to predilate the valve in order to enhance transcatheter delivery. TAVI has emerged as a ground‐breaking, minimally invasive alternative to traditional open‐heart surgery, primarily designed for aged patients initially considered unsuitable for surgical intervention due to severe aortic stenosis. Thus, the growing prevalence of aortic valve stenosis worldwide drives the balloon aortic valvuloplasty market growth.
Balloon Aortic Valvuloplasty Market: Competitive Landscape and Key Developments
B Braun SE; TT Medical, Inc.; Balton; Becton Dickinson and Co; Edwards Lifesciences Corp; Balt; Venus MedTech HangZhou Inc.; NuMED; simeks; and OSYPKA are a few key companies operating in the market. These companies focus on product innovation strategies to meet evolving customer demands, along with maintaining their brand name in the balloon aortic valvuloplasty market.
As per company press releases, a few recent developments initiated in the global balloon aortic valvuloplasty market report are mentioned below:
- In January 2023, Abbott received FDA approval for the Navitor TAVI system to treat patients with severe aortic stenosis and those who are ineligible for open-heart surgery. Navitor has been newly introduced in the company’s extensive transcatheter structural heart portfolio; it offers physicians and patients less invasive treatment alternatives for a range of serious heart conditions.
- In March 2021, Keystone Heart, Ltd. entered into an exclusive distribution agreement with InterValve Medical Inc. to sell and market their portfolio of balloon aortic valvuloplasty products in the US. Under the agreement, Keystone Heart Ltd. began selling the V8 and TAV8 aortic valvuloplasty balloon catheters (InterValve Medical) in the US market.