Peripheral Liquid Embolic Agents Market
Developments in Temperature-Induced Phase Change Systems and pH-Triggered Embolic Agents Drive Market Growth
Temperature-sensitive polymer solutions can change from a sol-injectable state to a gel state at room temperature in the body. Poly(N-isopropyl acrylamide) (PNIPAAm) exhibits a reversible phase transition in response to a lower critical solution temperature (LCST) of 32°C. According to a study published in the Journal of Controlled Release by Qian et al. in 2015, poly(N-isopropylacrylamide-co-butylmethyl acrylate) gels with iohexol dispersions for TACE of liver cancer demonstrated effective embolization of renal arteries in rabbits and sustained release of doxorubicin. Similarly, as per a study published in the National Library of Medicine by Wang et al. in 2011, chitosan/β-glycerophosphate (C/GP) successfully closed renal arteries in rabbits, indicating to be a potential embolization agent. Likewise, Zhao et al. also investigated temperature-sensitive PNIPAAm-iohexol nano gels for embolization, according to a research study published Wiley Online Library in 2011. The hydrophobic interactions above the phase transition temperature (36.5 °C) lead to a hierarchical 3D gel network for in situ vessel casting. Compared to Lipiodol, a peripheral embolic agent, the nanogel provided better peripheral embolization in VX2 liver tumors in rabbits.
Furthermore, a poloxamer 407 (a triblock copolymer of polyethylene oxide a–polypropylene oxide b–polyethylene oxide a) containing a thermosensitive liquid embolic hydrogel has been proposed for liver cancer therapy. This composite hydrogel composed of poloxamer 407, hydroxymethyl cellulose, sodium alginate, and iodixanol (PSHI) presented two phases with increasing temperature: a flowing sol and a shrunken gel. Notably, complete occlusion of the VX2 liver cancer model was demonstrated with PSHI-Ca2+ as the tumor disappeared after embolization.
pH-sensitive active ingredients undergo a phase transition due to the pH change between the in vitro and physiological environments. These hydrogels swell and collapse in response to pH, which is used to control drug release.
As per a study published in the Royal Society of Chemistry by Nguyen et al. in 2016, PCLA-PUSSM, a copolymer consisting of poly(ε-caprolactone-co-lactide) (PCLA), PEG, and poly(urethane sulfide sulfamethazine) (PUSSM) was developed, for TACE of HCC. This biodegradable material was loaded with doxorubicin and exhibited a phase transition in response to decreasing pH. The hydrogel successfully embolized a liver tumor from a VX2 rabbit tumor model while providing a sustained release of doxorubicin. In a rat model, embolization stopped blood supply to the tumor, creating an acidic environment through hypoxia and inducing lactic acidosis. The low pH triggered drug release and successfully inhibited tumor growth in vivo.
Thus, the growing focus and developments on temperature-induced phase change systems and pH-triggered embolic agents are expected to bring new trends in the peripheral liquid embolic agents market in the coming years.
Peripheral Liquid Embolic Agents Market: Application Overview
The peripheral liquid embolic agents market, based on application, is segmented into endoleak type II, malformations, bleedings and trauma, varicose vein, tumors, and visceral aneurysm. The endoleak type II segment held the largest peripheral liquid embolic agents market share in 2023. However, the bleedings and trauma segment is anticipated to register the highest CAGR during 2023–2031.
Type II endoleak (T2E) is the retrograde filling of the aneurysm sac by open aortic branch vessels, usually lumbar or inferior mesenteric arteries. T2E is the most common complication after endovascular aneurysm repair (EVAR). There is a shift toward liquid emboli, particularly NBC (N-butyl cyanoacrylate) and EVOH-based liquids such as Onyx (Onyx LES, Covidien), which are most commonly used. Iodized liquid polyvinyl alcohol (PVA) polymer agent (EASYX, Qmedics AG) with CE approval for peripheral use consists of a PVA polymer in liquid form that forms a solid cast when the solvent (DMSO) comes into contact with the blood circulation dissolves.
Peripheral Liquid Embolic Agents Market: Geographic Overview
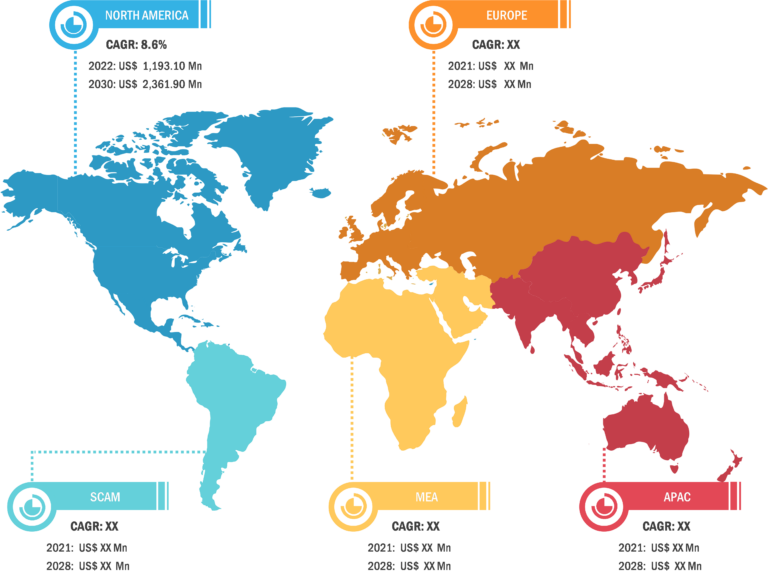
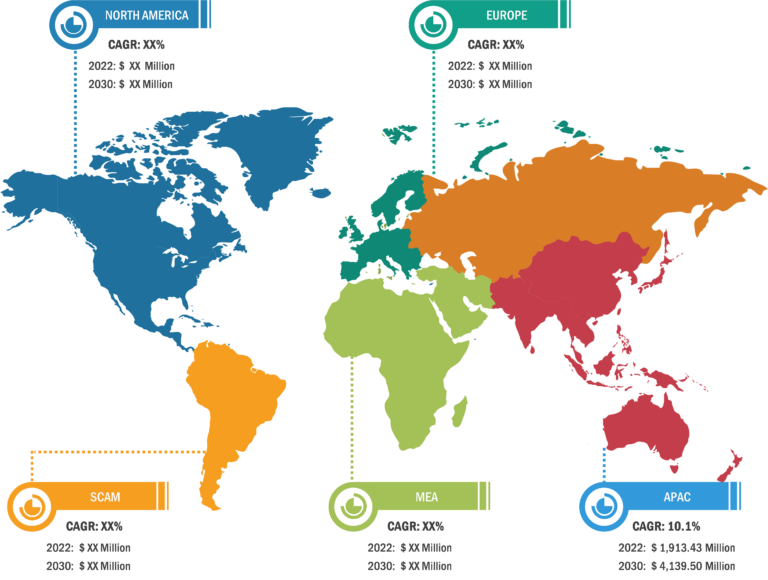
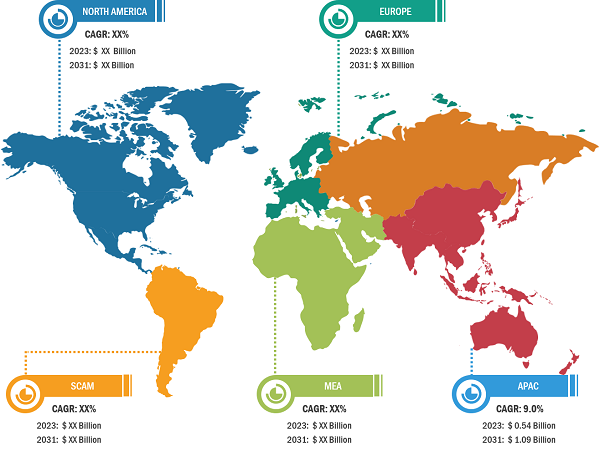
In terms of geography, the peripheral liquid embolic agents market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2023, North America accounted for the largest share of the market. The market growth in North America is attributed to the increasing cases of aneurysms, trauma, and others across the region. The market in Canada is anticipated to grow because of rising government support for addressing the increasing concerns regarding peripheral disorders. The market in Mexico is estimated to grow owing to the growing pharma industries and medical tourism across the country. Asia Pacific is expected to record the highest CAGR during 2023–2031. China held the largest market share in 2023, and the market in India is expected to show a significant growth rate in Asia Pacific. China, as a significant low- and middle-income country, faces challenges regarding peripheral artery disease (PAD) burden. According to the Journal of Vascular Surgery, in China, the total number of people affected by PAD is estimated to have increased by 40% from 2000 to 2020. China has the largest population with type 2 diabetes mellitus (T2DM). Type 2 diabetes affects over 116 million adults in China, and estimates predict that this number will rise to 147 million by 2045. People affected by type 2 diabetes are 1.5 to 4.0 times more likely to develop PAD than non-diabetic people. In China, the prevalence of PAD in diabetic patients has been reported to be between 6% and 10%. In India, the demand for embolization products is increasing because of the rising prevalence of peripheral diseases. In India, PAD is one of the underestimated and undertreated atherosclerotic vascular diseases. According to the article “The Indian Consensus Statement for the Management of Lower Extremity Peripheral Artery Disease” published in the Journal of Indian College of Cardiology, the prevalence of PAD varies between 7.6% and 26.7%. The etiological aspects, diagnostic approaches, treatment modalities, and other preventive measures in India vary in different regions.
Industry Developments and Future Opportunities:
As per company press releases, a few initiatives taken by leading players operating in the peripheral liquid embolic agents market are listed below:
- CERENOVUS Inc., launched TRUFILL n-BCA Liquid Embolic System Procedural Set. The latest addition to the CERENOVUS hemorrhagic stroke portfolio offers TRUFILL n-BCA Liquid Embolic System as a new procedural set that includes two configurations and the accessories needed to prep and deliver n-BCA Liquid Embolic System in one sterilized set, creating streamlined procedure preparation. (Johnson & Johnson, Press Release, March 2024)
- Sirtex Medical (“Sirtex”), launched first-of-its-kind product to treat peripheral vascular hemorrhage. LAVA provides volume and viscosity options that optimize the flexibility to treat patients with controlled target vessel occlusion. The product’s ability to maximize the packing density within the target vessel is incredibly important, as it allows us to minimize the likelihood of a future recurrence or restoration of vessel patency,. (Source: Sirtex Medical, Press Release, October 2023)
Peripheral Liquid Embolic Agents Market: Competitive Landscape and Key Developments
Medtronic, B Braun SE, Guerbet SA, Sirtex Medical Ltd, Balt, MicroVention Inc, Johnson & Johnson, and GEM srl are a few leading companies operating in the peripheral liquid embolic agents market. The players focus on expanding and diversifying their market presence and acquiring a novel customer base, thereby exploiting attractive business opportunities prevailing in the market.