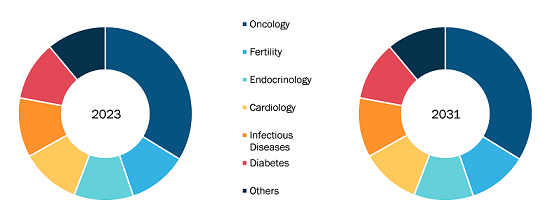
Crohn’s Disease Therapeutics Market
Growing Number of Drug Developments and Regulatory Approvals Drives Crohn’s Disease Therapeutics Market Growth
The majority of the established as well as new players are making efforts to manufacture drugs against Crohn’s disease. In 2023, the US Food and Drug Administration (FDA) approved the first pill—Rinvoq (upadacitinib)—to treat moderate-to-severe Crohn’s disease in adults who are irresponsive to drug candidates blocking tumor necrosis factor (TNF). Skyrizi (risankizumab-rzaa) is a monoclonal antibody (mAb) indicated for the treatment of moderate-to-severe plaque psoriasis, active psoriatic arthritis (PsA), and moderate-to-severe active Crohn’s disease (CD) in adult patients. The FDA approved Skyrizi for the treatment of adults with active PsA in January 2022 and for the treatment of adults with moderate-to-severe active Crohn’s disease in June 2022. The same drug received approval in Canada for the treatment of moderate-to-severe Crohn’s disease in adults in October 2022. The European Commission (EC) approved Skyrizi for the same indication in November 2022. The drug is jointly developed and marketed by AbbVie and Boehringer Ingelheim under an agreement that was signed in March 2016.
North America held the largest share of the global Crohn’s disease therapeutics market in 2023, owing to the increasing number of cases in the US. Furthermore, the increasing median age population and the presence of key market players involved in product development benefit the market in this region. The US held the largest share of the Crohn’s disease therapeutics market in North America in 2023. Crohn’s disease is mainly characterized by inflammation and irritation in the digestive tract. As per the National Center for Chronic Disease Prevention and Health Promotion, 6 in 10 people in the country have at least one chronic disease. The American Hospital Association estimates that ~133 million people have at least one chronic disease, and the number is expected to reach 170 million by 2030. As per Crohn’s and Colitis Foundation, it is estimates nearly 1 in 100 Americans have inflammatory bowels diseases and the same research also shows an estimated 2.4 million Americans are suffering some form of IBD (irritable bowel syndrome). Various factor like ultra processed food, pollution and industrial hazardous chemical are responsible for the risk of getting IBD. The median age i.e. in between 30 to 40 are most prone to suffering from Crohns diseases and its decreases later with age. The Canadian Digestive Health Foundation states millions of Canadians live with digestive disease. ~20 million Canadians, i.e., 2 out of every 3 people, suffer from digestive disorders every year. Thus, the increasing prevalence of IBD in North America fuels the growth of the Crohn’s disease therapeutics market.
Crohn’s Disease Therapeutics Market: Competitive Landscape and Key Developments
Abbvie, Inc.; Johnson and Johnson Private Limited; GlaxoSmithKline Plc; Merck and Co., Inc.; Novartis Ag; Celgene Corporation; Genentech; Pfizer Inc.; Nestle Health Science; and Boehringer Ingelheim GmbH are a few major companies operating in the Crohn’s disease therapeutics market. Market players adopt product innovation strategies to meet evolving customer demands, thereby maintaining their brand names in the market.
A few strategic plans by key players operating in the Crohn’s disease therapeutics market are listed below:
In April 2022, Microbiotica, a leading player in microbiome-based therapeutics and biomarkers, announces it has received project funding from the Crohn’s & Colitis Foundation, as part of their IBD Ventures program, for the development of therapies to treat inflammatory bowel disease (IBD). The funding will be used to progress the development of MB310 through cGMP manufacturing to enable an Investigational New Drug (IND) application, allowing progression into a first-in-human clinical trial.
In 2022, Engitix Ltd, a biotechnology company developing a portfolio of programmes in fibrosis and solid tumours using its proprietary human extracellular matrix (ECM) platform, entered into an agreement with Takeda to expand an existing collaboration to include the discovery and development of novel therapeutics for fibrostenotic inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis. Under the terms of this agreement, Engitix and Takeda will collaborate in the confirmation and validation of targets and the preclinical development of therapeutics in IBD using Engitix’s unique extracellular matrix (ECM) discovery platform.