Strategic Initiatives by Manufacturers Drive Rapid Test Kits Market
In recent years, molecular methods such as PCR have evolved as more effective diagnostic tools, as they aid in the precise diagnosis of a wide range of infectious diseases. Most of the popular molecular tests use reverse transcription polymerase chain reaction (RT-PCR). The technique provides reproducible results that are comparable between different laboratories and hence are accepted worldwide. Manufacturers are also focused on developing new and advanced models of PCR instruments. GeneSoC is a recently developed system in which the PCR reaction is reciprocally driven over numerous preheated zones and encapsulated on a chip within an extremely thin flow channel. GeneSoC is available as multiplex real-time PCR and is capable of concurrently detecting 3 fluorescence channels. It is regarded as an excellent alternative for the diagnosis of influenza A and B viruses, as it completes the real-time RT-PCR reaction in ~16 minutes.
PCR techniques are also being widely accepted and utilized in emerging countries. Moreover, in October 2021, Thermo Fisher Scientific introduced the Applied Biosystem Quantstudio Absolute Q-Digital PCR System, the first fully integrated dPCR system designed to deliver highly accurate and consistent genetic analysis. The company claims that the product generates research results within 90 minutes. Furthermore, in May 2022, Bio-Rad Laboratories, Inc. introduced its CFX Duet Real-Time PCR System in selected countries that accept the CE Mark. The system is intended to support researchers in developing singleplex and duplex qPCR assays.. Thus, the rapid test kits market size is likely to surge by 2030 owing to the growing preference for PCR as a diagnostic tool.
Rapid Test Kits Market: Regional Overview
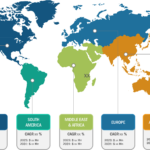
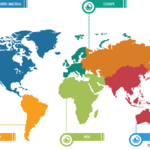
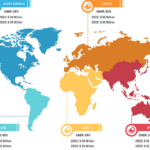
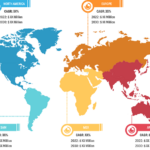
In terms of geography, the rapid test kits market is segmented into North America, Europe, Asia Pacific, South & Central America, and the Middle East & Africa. In 2022, North America held the largest share of the rapid test kits market. The market is fueled by the increasing demand for innovative products from bio-analytical instrument manufacturers. The increasing focus on incorporating advanced diagnostic methods in government-aided and private healthcare facilities promotes the adoption of rapid test kits.
The US rapid test kits market growth is further fueled by a rapid increase in at-home test use occurred between the SARS-CoV-2 Delta- and Omicron-predominant periods of the pandemic. According to the CDC, from late 2020 till May 2022, more than 70 million test kits were shipped across the US.
The rapid test kits market in Asia Pacific is expected to register the highest CAGR during the forecast period. Market growth in the region is attributed to a surge in the use of antigen testing methods due to a rise in the number of cases of infectious diseases. Influenza, hepatitis B, AND infectious diarrhea are among the common infectious diseases in China, while India records typhoid, malaria, jaundice, tuberculosis, and AIDS cases in huge numbers. The WHO considers HIV self-testing (HIVST) to be a legitimate alternative to standard HIV testing performed in diagnostic laboratories. Over the last 5 years, the healthcare sector and authorities in China have strived to gradually increase the extent of HIVST use across the country. The coverage of HIV testing can be further increased with the help of HIVST to identify the condition among individuals suffering from HIV but are ignorant of their infection.
Rapid Test Kits Market: Competitive Landscape and Key Developments
Apart from factors driving the market, the rapid test kits market report emphasizes prominent players operating in the market; these include F. Hoffmann-La Roche Ltd, Becton Dickinson and Co, ARKRAY Inc, Sysmex Partec GmbH, Fujirebio Europe NV, bioMerieux SA, Cepheid, Meril Life Sciences Pvt Ltd, QIAGEN NV, OraSure Technologies Inc, Guangzhou Wondfo Biotech Co Ltd, Denka Co Ltd, Abbott Laboratories, Trinity Biotech Plc, SD Biosensor Inc, Bio-Rad Laboratories Inc, Hologic Inc, DiaSorin SpA, Premier Medical Corp Pvt Ltd, and Beckman Coulter Inc. Market players focus on expanding and diversifying their businesses, and acquiring novel customer bases, which allows them to explore attractive business opportunities prevailing in the rapid test kits market.
In June 2021, DiaSorin SpA introduced the LIAISON Murex AntiHEV IgG & IgM test, the first fully automated high-throughput CLIA test for the diagnosis of hepatitis E (HEV). The test was made available on LIAISON platforms in all countries that recognize the CE mark.
In November 2022, OraSure Technologies launched its InteliSwab COVID-19 Rapid Test, which is available on Amazon online store in the US. The test is simple and easy to use, as it requires only a swab of the lower nostrils and a swirl of a pre-measured solution..
In January 2022, Roche Diagnostics India, a part of F. Hoffmann-La Roche Ltd, announced the launch of the COVID-19 At-Home Test. The newly launched diagnostic solution is approved by the Indian Council of Medical Research (ICMR). The at-home test kit can detect SARS-CoV-2 virus, including the Omicron variant. The kits comprise a sterile swab, a test cassette, a tube with liquid, and a nozzle cap, along with a self-test guide and QR code to access the instruction video. Users can also receive help reading and interpreting the test results and updating them on the ICMR database upon scanning the QR code.
In November 2021, MedAccess, Clinton Health Access Initiative, and SD Biosensor partnered to make the STANDARD Q HIV/Syphilis Combo test available for less than US$ 1 in over 100 low- and middle-income countries. This dual rapid test for syphilis and HIV I a point-of-care diagnostic tool that enables the simultaneous diagnosis of both diseases in under 20 minutes from a single finger-prick sample. This partnership, enabled by a volume guarantee agreement, reduces the price of the STANDARD Q test by over 32%, benefiting nearly 6 million pregnant women in high-burden countries.