Antibody Drug Conjugates Market
Escalating Pipeline of ADCs Would Lead to Future Trends in Market
Various biopharmaceutical companies are developing ADCs owing to the rising interest in ADCs as promising cancer treatment. According to an article, “Antibody-Drug Conjugates Clinical Trial Pipeline Experiences Momentum,” published in June 2023, over 300 ADCs are under development with the involvement of more than 180 companies. It is anticipated that the commercial launch of the ADCs developed will significantly enhance market size. Companies such as ImmunoGen, Inc.; NBE-Therapeutics, Seagen Inc; ADC Therapeutics; Merck KGaA; Sorrento Therapeutics, Inc; Mythic Therapeutics; Sutro Biopharma; Peak Bio; Regeneron Pharmaceuticals; Asana BioSciences; Sanofi; Navrogen, Inc; Tanabe Research Laboratories USA; and OBI Pharma have ADCs in the different developmental phases. The companies have developed a broad list of promising ADCs, which are in different clinical stages or in pipelines to be commercialized. The list of ADCs in the pipeline includes Camidanlumab tesirine, ADCT-602, Zynlonta, ADCT-901, ADCT-701, ADCT-212, ADCT-601, Ladiratuzumab Vedotin, NBE-002, IMGN151, IMGN-632, IMGC 936, IMGN-151, OBI 999, R 992, NAV-001, MYTX-011, M1231, STI-6129, Torpedo, BCMA ADC, REGN5093-M114, ASN004, and TR 1801 ADC.
Several antibody drug conjugates market players have also shared pre-clinical data related to their respective products. For instance, in April 2023, Mythic Therapeutics announced preclinical data for its MYTX-011 (cMET-targeting ADC). The data published included the highlights of MYTX-011 intended to treat a broad range of cMET+ cancers. The preclinical information was presented at the American Association for Cancer Research Annual Meeting. Likewise, at the Association for Cancer Research Annual Meeting in April 2023, Araris Biotech AG announced its preclinical data on anti-Nectin-4 and anti-HER2 ADCs. The two ADCs were developed using the company’s proprietary linker technology. The data demonstrated improved antitumor activity of anti-Nectin-4 and anti-HER2 ADCs compared to respective FDA-approved ADCs in head-to-head in-vivo studies. Thus, a rise in innovative ADCs is likely to propel the market growth exponentially in the coming years.
Antibody Drug Conjugates Market: Segmental Overview
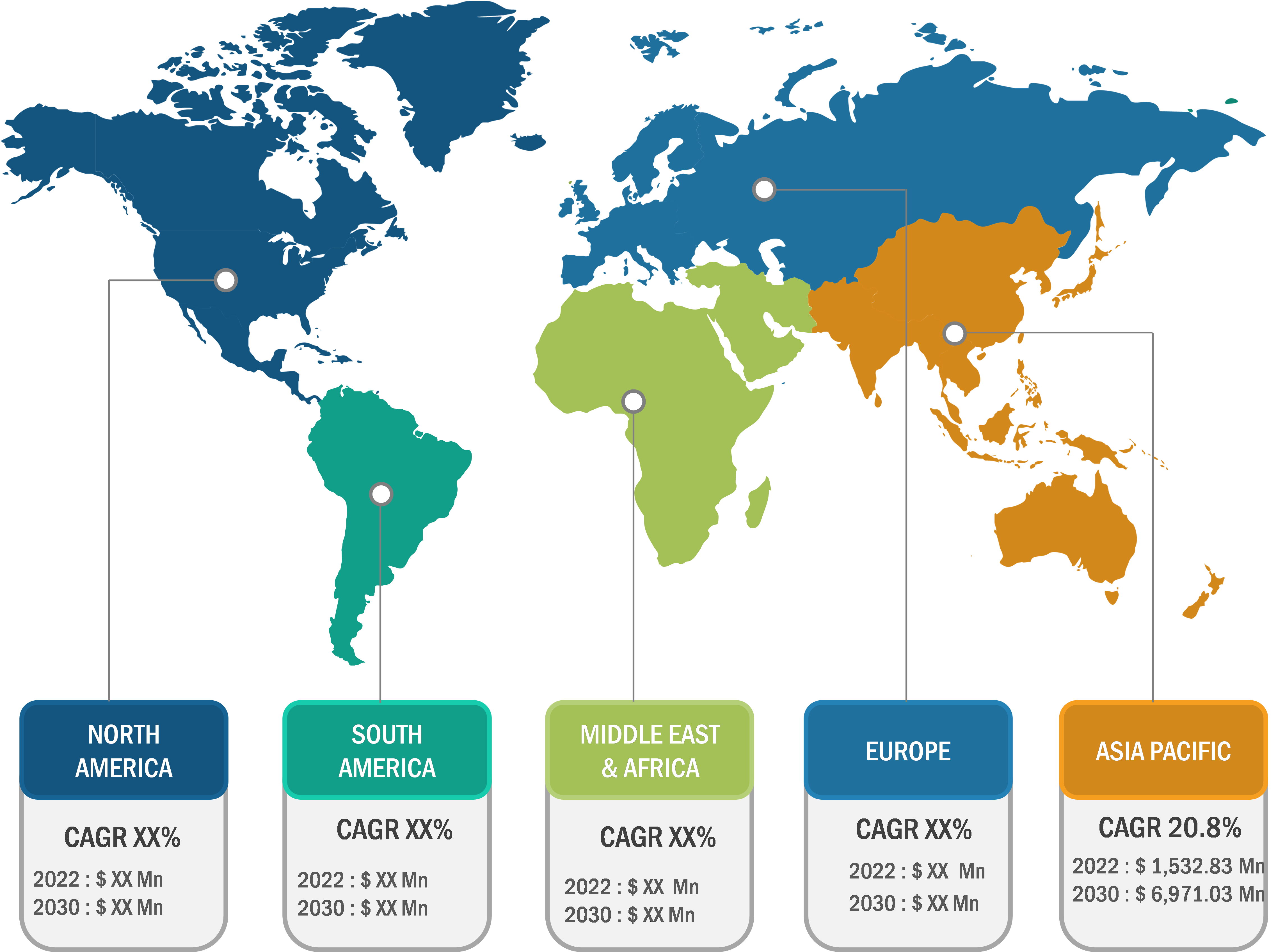
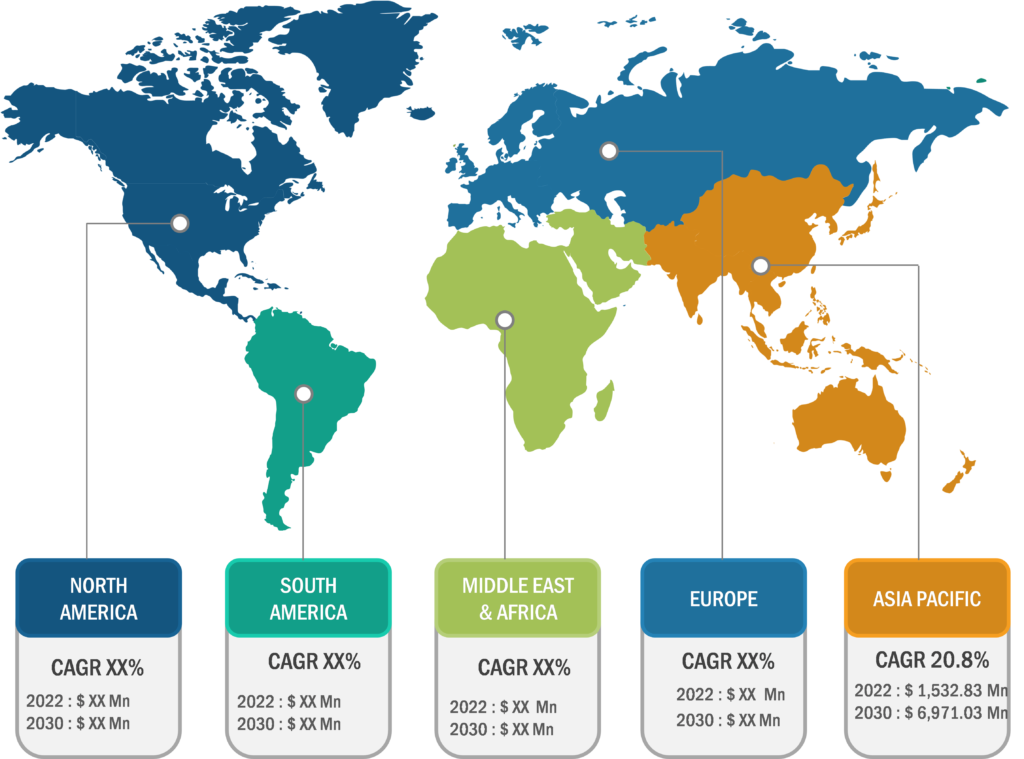
The antibody drug conjugates market is segmented on the basis of technology, application, distribution channel, and geography. The market, by technology, is bifurcated into cleavable linkers and non-cleavable linkers. In 2022, the cleavable linkers segment held a larger market share, and the non-cleavable linkers segment is estimated to register a faster CAGR during 2022–2030.
Antibody Drug Conjugates Market: Competitive Landscape and Key Developments
ADC Therapeutics SA; Pfizer Inc; F. Hoffmann-La Roche Ltd; ImmunoGen, Inc; GSK Plc; Gilead Sciences Inc; AstraZeneca Plc; Astellas Pharma Inc; RemeGen Co Ltd, and Takeda Pharmaceutical Co Ltd. are among the leading companies operating in the antibody drug conjugates market. These players focus on diversifying and expanding their market presence alongside acquiring a novel customer base, thereby tapping prevailing business opportunities in the antibody drug conjugates market.
Market players are launching new products into the market. A few of the examples of such new launches as mentioned below:
- In August 2023, ADC Therapeutics SA announced that two ZYNLONTA (loncastuximab tesirine-lpyl) abstracts have been accepted for presentation at the Eleventh Annual Meeting of the Society of Hematologic Oncology (SOHO 2023). The data show signs of durable responses in patients suffering from refractory or relapsed diffuses large B-cell lymphoma.
- In July 2023, Gilead Sciences, Inc. received approval from the European Commission (EC) for its Trodelvy (sacituzumab govitecan) as a monotherapy for treating adults suffering from unresectable or metastatic hormone receptor (HR)-positive HER2-negative breast cancer.
- In May 2020, Takeda China announced that ADCETRIS (brentuximab vedotin) was officially approved by China’s National Medical Products Administration (NMPA) for use in adults with relapsed or refractory systemic anaplastic large cell lymphoma (sALCL) or CD30-positive Hodgkin lymphoma.