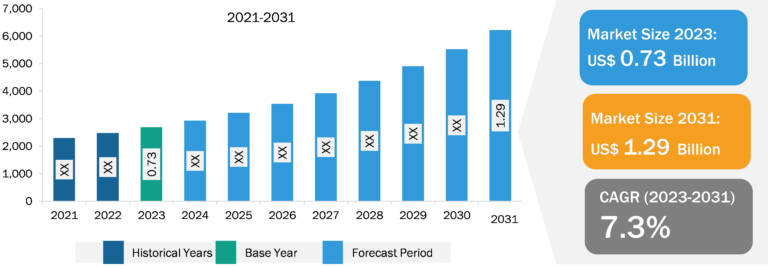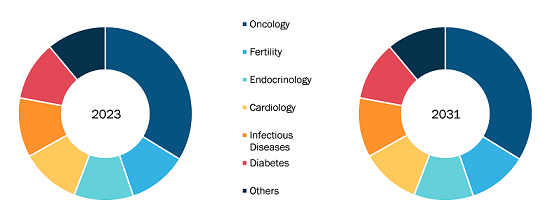
Transdermal Drug Delivery System Market
Asia Pacific is expected to record the fastest CAGR in the global transdermal drug delivery system market during 2022–2030. North America accounted for the largest share of the market in 2022. The US held the largest share of the transdermal drug delivery system market in North America in 2022. According to the American Heart Association (AHA), cardiovascular disease (CVD) remains the leading cause of death in the US. The CDC states that CVD accounted for ~695,000 deaths, i.e., ~1 in 5 deaths in the US in 2021. The rising prevalence of cardiovascular disease, diabetes, chronic pain conditions, and other chronic diseases drives the demand for effective and convenient treatment options. Medical patches offer a noninvasive and patient-friendly approach to deliver medication and manage symptoms associated with these chronic conditions. Ongoing advancements in TDDS, such as wearable sensors and flexible electronics are likely to contribute to the growth of the transdermal drug delivery system market in the coming years. These advancements allow for more accurate dosing, enhanced patient monitoring, and improved patient compliance. According to the report “Recent advances in transdermal drug delivery systems,” published by Biomaterials Research in 2021, microneedles attract significant attention among TDDS modalities, which overcome the limitations of the existing simple application type and patch-type needles by combining the advantages of microneedles to obtain higher treatment efficiency and effects. Advancements in these TDDS are likely to result in means for better control of the prevalence of cardiovascular and central nervous system diseases, diabetes, neuromuscular diseases, and infectious and localized infectious diseases.
The growing trend of personalized medicines, where treatments are tailored to individual patients, has boosted the development of medical patches that offer controlled and targeted drug delivery. This customization allows for better patient outcomes and improved therapeutic efficacy. In 2020, Reddy’s Laboratories launched an authorized generic version of the NitroDur (nitroglycerin) transdermal infusion system in the US. With the growing emphasis on home healthcare and remote patient monitoring, medical patches offer a convenient solution for delivering medications and monitoring vital signs outside traditional healthcare settings.
The US transdermal drug delivery system market is influenced by factors such as the increasing prevalence of chronic diseases, rising technological advancements, and growing focus on personalized medicines.
Rising Demand for Noninvasive Drug Delivery Devices Drives Transdermal Drug Delivery System Market Growth
Traditional drug delivery techniques such as intramuscular, intravenous, and rectal drug administration are not preferred by certain patient populations mainly due to the pain and complexities associated with them. With improvements in medical science and definite drug delivery technologies, noninvasive drug delivery systems are now set to compete with the traditional method of injectable route of drug administration. The noninvasive drug delivery systems include drug delivery via oral; topical; transdermal-active (device-aided enhanced penetration) and transdermal-passive; and transocular membrane, transmucosal membrane, and alveolar membrane from inhaled medication. Transdermal drug delivery is advantageous in many ways over the oral route of administration. In particular, it evades the first-pass mechanism of the liver, which can otherwise impulsively metabolize drugs.
Further, transdermal delivery is not as painful as hypodermic injections, and this route leads to no dangerous medical waste and poses no risk of disease transmission by needle reuse, which is especially common in developing and under-developed countries. In addition, a noninvasive transdermal drug delivery system has simplified dosing schedules, and the drugs can be self-administered. Patient compliance is much higher when drugs are administrated noninvasively due to low or no pain involved. Therefore, this route is considered a preferred mode of drug delivery. Moreover, a noninvasive drug delivery system can significantly reduce the cost of clinical use because of the self-administration of the drugs by the patients. Thus, the advantages associated with a noninvasive drug delivery facilitated by transdermal drug delivery systems drive the market growth.
Transdermal Drug Delivery System Market: Segmental Overview
The global transdermal drug delivery system market is segmented on the basis of product, application, and distribution channel. Based on product, the market is segmented into transdermal patches, transdermal gels, transdermal sprays, and others. The transdermal drug delivery system market, by application, is divided into cardiovascular diseases, central nervous system disorders, pain management, hormones applications, and other applications. Based on distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, online pharmacies, and others.
Transdermal Drug Delivery System Market: Competitive Landscape and Key Developments
Novartis AG, Johnson & Johnson, GSK Plc, Viatris Inc, Boehringer Ingelheim International GmbH, Luye Pharma Group Ltd, Hisamitsu Pharmaceutical Co Inc, Lavipharm SA, Purdue Pharma LP, and UCB SA are among the key companies operating in the transdermal drug delivery system market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the transdermal drug delivery system market.
A few of the recent developments in the global transdermal drug delivery system market are mentioned below:
- In October 2023, Novartis completed a 100% spin-off of Sandoz, its Generics and Biosimilars business, to create an independent company.
- In December 2022, Luye Pharma Group held an offline product launch conference in Shanghai, along with an online event, to announce the launch of Ruoxinlin (Toludesvenlafaxine hydrochloride extended-release tablets) in China. Ruoxinlin is China’s first class-1 innovative chemical drug independently developed for the treatment of major depressive disorder (MDD) through a local administration and protected by intellectual property rights. The drug provides a powerful new option for patients, bringing the treatment of MDD to a new level.
- In February 2021, Luye Pharma Switzerland AG, a subsidiary of Luye Pharma Group, entered into an agreement with Towa Pharmaceutical Co Ltd (Towa). The terms of this agreement grant Towa exclusive rights to the development and commercialization of Rivastigmine Multi-Day (Rivastigmine MD) transdermal patch in Japan. The patch indicates a twice-weekly transdermal application to treat Alzheimer’s disease. Luye Pharma has applied for several foreign patents and has been granted several ones to protect this product. Rivastigmine MD is presently in the registration stage in Europe and will soon begin phase III clinical research there. Luye Pharma has provided Towa exclusive rights to create and market Rivastigmine MD in Japan.
- In January 2021, Luye Pharma Group held a press conference to announce the launch of its Rivastigmine Transdermal Patch in China. The medication is approved for the treatment of mild to moderate Alzheimer’s disease and should be used topically once daily. Luye Pharma AG, the company’s German affiliate, has created this patch using its unique transdermal patch technology. Luye Pharma AG sells Rivastigmine Transdermal Patch in the US, 11 European nations, and Thailand, among other nations, apart from China.