Vascular Closure Device Market
North America accounted for the largest share of the global vascular closure device market in 2022. Asia Pacific is expected to record the highest CAGR in the market during 2022–2030. The US held the largest share of the vascular closure device market in North America. The US has a well-developed healthcare sector with access to highly advanced equipment and instruments. The country reports a high prevalence of cardiovascular diseases. According to the “Heart Disease and Stroke Statistics – 2023 Update” by the American Heart Association, in 2020, coronary heart disease (CHD) was a leading cause (41.2%) of deaths associated with CVDs in the US, followed by stroke (17.3%), other CVD (16.8%), high blood pressure (12.9%), heart failure (9.2%), and artery diseases (2.6%), respectively. As per the US Centers for Disease Control and Prevention (CDC), ~1 in 20 adults in the US, aged 20 and above, have coronary artery disease. The study also mentions coronary artery disease as the most common type of heart disease in the US. Thus, a high prevalence of CVDs and coronary artery disease would propel the adoption of vascular closure devices during surgical interventions in the US in the coming years.
The US is a hub for medical innovations and technological advancements in healthcare. Continuous advancements in technologies associated with minimally invasive procedures, improvements in closure device technologies, and the introduction of novel closure devices contribute to the growth of the vascular closure devices market in the US. For instance, in March 2023, Haemonetics Corporation (US) invested ~US$ 31.72 million (EUR 30 million) in Vivasure Medical Limited. In March 2023, Vivasure Medical announced FDA IDE approval to Initiate a US Pivotal Study evaluating the safety and effectiveness of the Vivasure PerQseal Closure Device System. In 2021, VASCADE MVP was the first and only FDA-approved vascular closure device indicated for use following atrial fibrillation (AF) ablation to allow same-day discharge.
Burgeoning Demand for Minimally Invasive Devices Drives Vascular Closure Device Market Growth
Improvements in medical science and technologies have given way to minimally invasive devices, which can aid in the replacement of traditional painful surgical methods by minimally invasive or noninvasive methods. Minimally invasive techniques have gained popularity in surgical fields such as cardiac surgery. Many of these procedures are performed using femoral cannulation for extracorporeal circulation. Vascular closure devices are mainly used in transcatheter and endovascular interventions to enable wound healing and reduce procedure time.
Angiography is one of the common procedures performed in a wide variety of specialties, including vascular surgery, interventional radiology, and diagnostic and interventional cardiology. Vascular closure devices are useful in the settings of a large body habitus, anticoagulation, and antiplatelet therapies. They are also used in cases where extended bed rest may not be desirable (e.g., in patients with extensive pressure ulcers). A vascular closure device with resorbable collagen material, which eliminates the use of suture materials, has been introduced for transfemoral transcatheter aortic valve implantation (TAVI). For instance, the MANTA device by Teleflex Medical Inc. is adequate for the closure of arterial access sites of up to 25 Fr. Safety and efficacy of the system were demonstrated in a real-world TAVI patient cohort. Thus, the increasing demand for minimally invasive devices drives the vascular closure devices market growth.
Vascular Closure Device Market: Segmental Overview
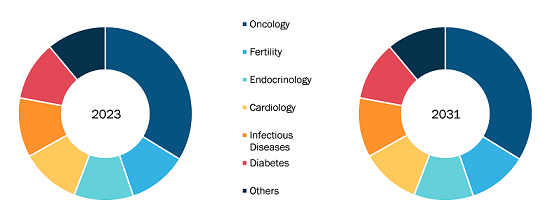
The global vascular closure device market is categorized on the basis of product type, access, procedure, and end user. Based on product type, the market is divided into active approximators, passive approximators, and external hemostatic devices. In terms of access, the vascular closure device market is divided into femoral access and radial access. Based on procedure, the market is segmented into interventional radiology, interventional cardiology, and endovascular surgical. The vascular closure device market, by end user, is segmented into hospitals, ambulatory surgical centers, and others.
Vascular Closure Device Market: Competitive Landscape and Key Developments
Cordis Corp, Transluminal Technologies LLC, Vasorum Ltd, Haemonetics Corp, Abbott Laboratories, Becton Dickinson and Co, Cardinal Health Inc, ENDOCOR GmbH & CO KG, Medtronic Plc, and Teleflex Inc are a few key companies operating in the vascular closure device market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the vascular closure device market.
A few of the recent developments in the global vascular closure device market are mentioned below:
- In August 2023, Haemonetics Corp announced that its VASCADE MVP Venous Vascular Closure system was used for the first time in Germany in treatments. This marked the first use of VASCADE systems, which are used widely in hospitals in the US, in a European country.
- In June 2023, Becton Dickinson and Co. opened a new manufacturing facility in Tijuana. The new 15,775-square-meter facility produces devices and other products to improve medication safety within healthcare settings.
- In May 2023, Cardinal Health Canada planned to open a new distribution center in Greater Toronto, strategically expanding its distribution channel with the addition of 9 other locations to better meet the medical and surgical product demands of the Canadian healthcare system.
- In September 2022, Haemonetics Corp earned CE mark certification for its VASCADE vascular closure and VASCADE MVP venous vascular closure systems.
- In May 2022, Teleflex Inc, a global provider of medical technologies, received Health Canada approval for the MANTA Vascular Closure Device—the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial access site closure. This approval marks an important milestone in the plan of Teleflex to expand the availability of the MANTA globally, followed by apt regulatory approvals, thereby providing healthcare systems in Canada and in the world access to its another uniquely designed device.