Skin Cancer Treatment Market
Increasing R&D pipelines focused on skin cancer treatments, growing prevalence of skin cancer, and rising awareness of skin cancers drive the skin cancer treatment market growth. However, the high cost and adverse effects and problems of the treatment hinder the skin cancer treatment market growth.
The cases of skin cancer are rising even though the majority of these cases are avoidable. Risk factors of skin cancer include sunlight, tanning salons, sunlamps, and certain medical conditions or medications. This cancer type is mostly caused by UV radiation as it modifies the genetic material (DNA) in cells. The WHO predicted in March 2022 that there would be a more than 50% increase in the annual number of new cutaneous melanoma cases between 2020 and 2040.
The American Academy of Dermatology (AAD) started the SPOT Skin Cancer awareness initiative in May 2021. The program aims to increase awareness to help prevent skin cancer, identify it early, and save lives. Government initiatives to raise skin cancer awareness are anticipated to drive the skin cancer treatment market expansion in the coming years.
Increasing number of novel skin cancer therapies approved by worldwide regulatory bodies would propel the skin cancer treatment market growth during the forecast period. In February 2021, the US FDA approved the PD-1 inhibitor Libtayo (cemiplimab-rwlc) as the first immunotherapy recommended for patients affected by advanced basal cell carcinoma (BCC).
Adverse effects associated with existing treatment modalities negatively impact the skin cancer treatment market growth. Conventional treatment options such as surgery, chemotherapy, radiation therapy, and immunotherapy are often associated with adverse effects, including skin irritation, systemic toxicities, long-term complications, and others. The side effects can significantly impact patients’ quality of life and may deter some individuals from seeking or adhering to treatment. Moreover, the limitations of current treatment options may drive the requirement for alternative, less invasive, and more targeted therapies for skin cancer. As research and development activities focus on advancing treatment modalities with improved safety profiles and efficacy, the market is poised to witness transformative innovations that address these challenges, offering new hope for patients and driving the expansion of the skin cancer treatment market.
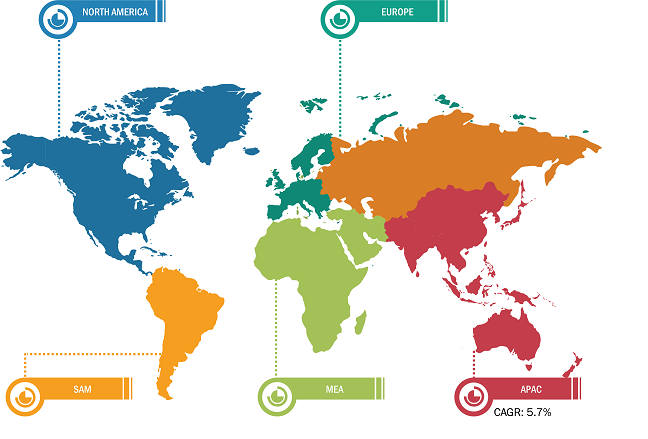
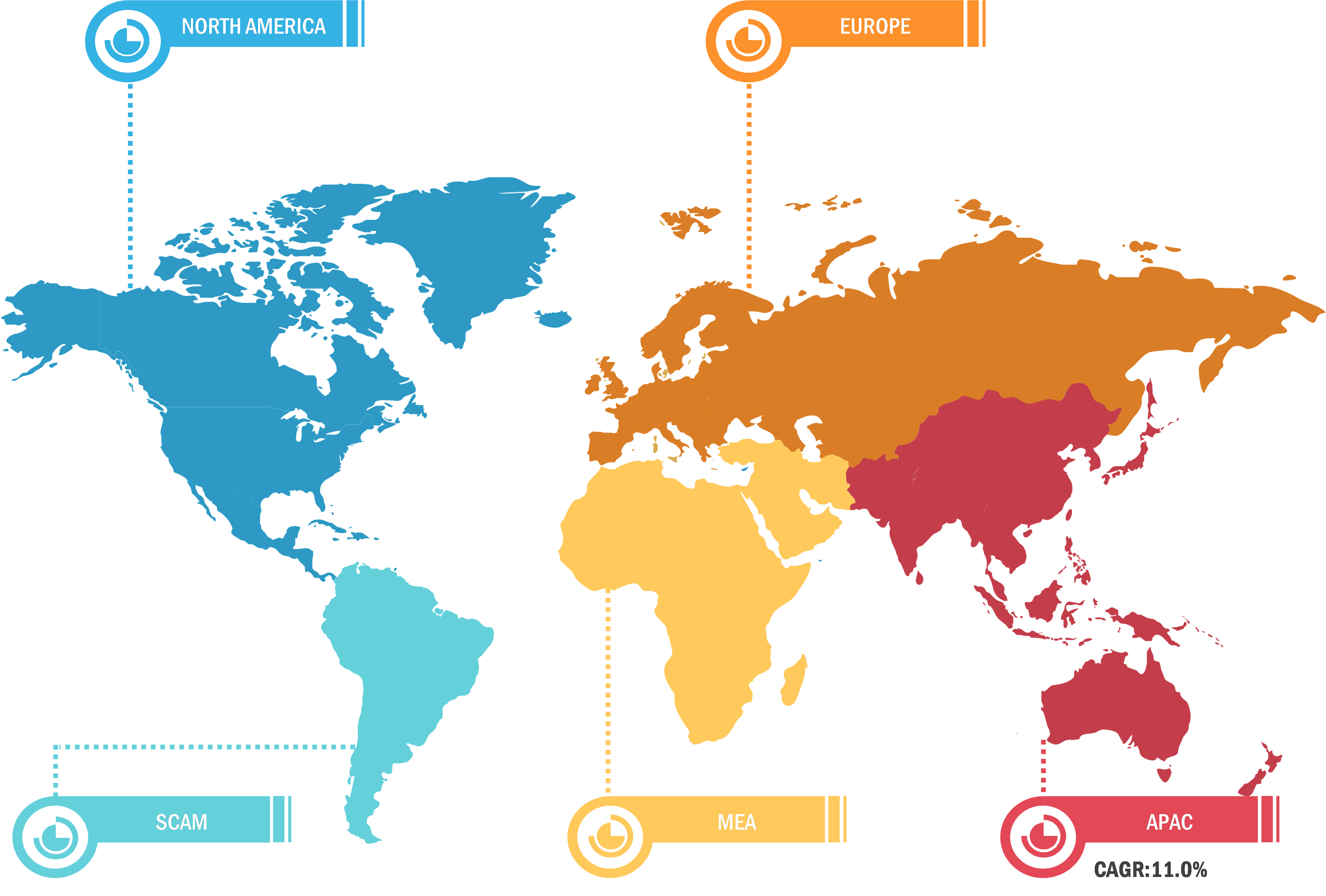
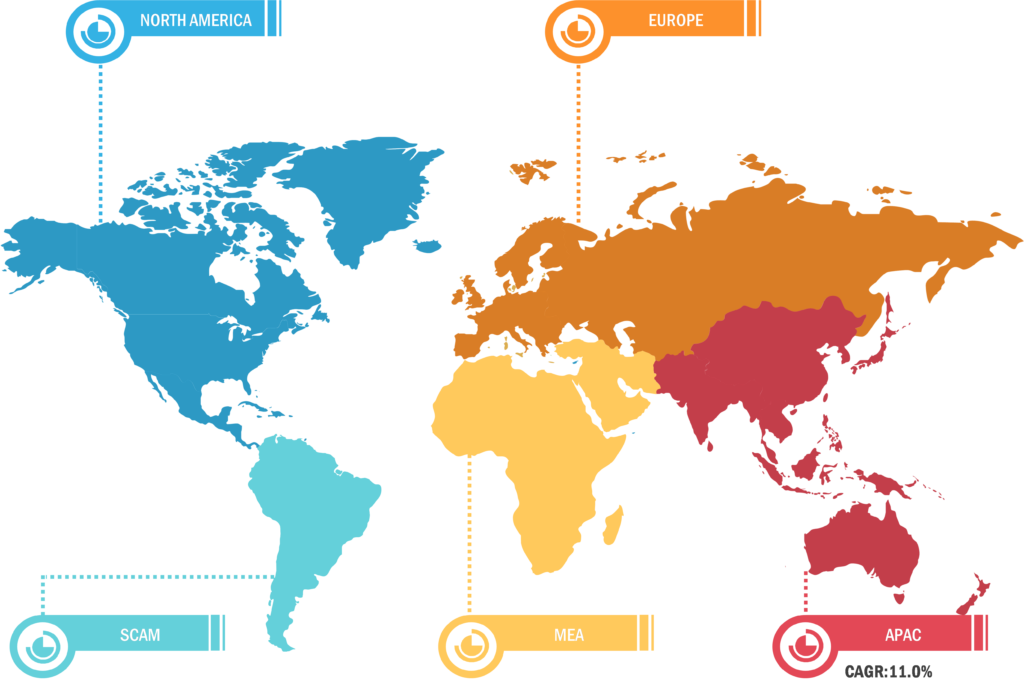
Based on region, the skin cancer treatment market is segmented into North America, Europe, Asia Pacific, the Middle East & Africa, and South & Central America. North America is the most significant contributor to the growth of this market owing to a higher incidence of skin cancer in the US and the greater uptake of cancer medications in that nation. In addition, the inclination of major industry participants to introduce novel treatments for skin cancer is predicted to have a favorable impact on the expansion of the market in the area. Because non-melanoma skin cancer is more common in Caucasian populations, North America and Europe account for a large portion of the global market.
Throughout the forecast period, the market in Asia Pacific is anticipated to grow at the fastest rate. The demand for skin cancer treatments is expected to rise in the Asia-Pacific area due to the rising prevalence of both melanoma and non-melanoma in nations like Australia and New Zealand. The combination of regulatory approvals for these skin cancer medications in the Asia Pacific and the sizeable and underdeveloped market in the area’s developing nations is expected to support the expansion of the skin cancer treatment market in that region. Because melanoma occurrences are rising in Europe, the market revenue is likewise expected to develop healthily during the forecast period.
Skin Cancer Treatment Market : Competitive Landscape and Key Developments
Merck & Co., Brristol Mayers Squibb, Novartis AG, Roche, Amgen Inc., GSK, Pfizer Inc., Sun Pharmaceutical, and Regeneron Pharmaceuticals are a few of the key companies operating in the skin cancer treatment market. Market players adopt product innovation strategies to meet evolving customer demands, maintaining their brand image in the skin cancer treatment market.
A few recent developments by skin cancer treatment players are mentioned below:
- In October 2023, With the US FDA’s approval of Opdivo (nivolumab), Bristol Myers Squibb can now treat adult and pediatric patients 12 years and older with completely resected stage IIB or IIC melanoma as an adjuvant. This expands Opdivo’s current indication and furthers the company’s legacy of offering melanoma patients treatment options.
- In March 2023, Zynyz (retifanlimab-dlwr), a humanized monoclonal antibody that targets programmed death receptor-1 (PD-1) was approved by the U.S. Food and Drug Administration for the treatment of adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). Based on tumor response rate and duration of response (DOR), the FDA approved Zynyz under accelerated approval; however, Zynyz’s continued approval for this indication may be subject to verification and a description of clinical benefit in confirmatory trials.
- In March 2023, the US FDA approved Zynyz, a humanized monoclonal antibody that targets programmed death receptor-1 (PD-1), for the treatment of metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). Based on tumor response rate and duration of response (DOR), the FDA approved Zynyz under accelerated approval; however, Zynyz’s continued approval for this indication may be subject to verification and a description of clinical benefit in confirmatory trials.
- In January 2022, Immunocore Holdings received US FDA approval for KIMMTRAK. It is used to treat adult patients suffering from HLA-A*02:01-positive uveal melanoma (mUM). Immunocore Holdings, a commercial-stage biotechnology company, is leading the development of a novel class of T cell receptor bispecific immunotherapies intended to treat an extensive range of diseases, including cancer, autoimmune, and infectious diseases.