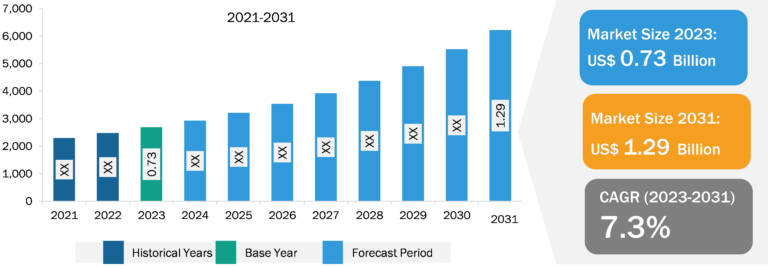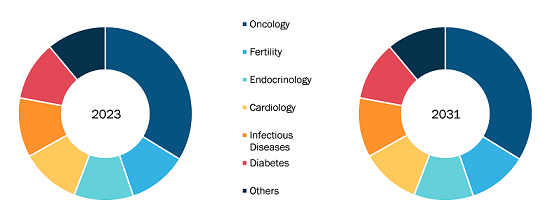
Glaucoma therapeutics Market
Glaucoma is a group of eye disorders caused due to the damage incurred to the optic nerve, which can eventually lead to total blindness. People with glaucoma may experience a gradual loss of nerve tissue, resulting in vision impairment or blindness if left untreated. Prostaglandin analogs are among the drugs widely recommended for effectively reducing eye pressure by promoting better drainage from the eye. As per the Glaucoma Research Foundation, in 2022, ~ 80 million were affected with glaucoma globally. In 2010, it was estimated that 8.4 million people worldwide had lost their vision due to primary open-angle glaucoma. According to the Glaucoma Research Foundation, the number is projected to increase to 22 million by 2040. Propelling incidences of glaucoma among the global population are a major driver of the glaucoma therapeutics market. The aging global population has also led to an increase in the incidence of glaucoma, creating a significant opportunity for the glaucoma therapeutics market growth. The demand for innovative therapies and management strategies is rising due to the higher susceptibility of the aging demographic to the disease. Moreover, the importance of regular eye check-ups for early detection and management of glaucoma has increased patient engagement and demand for advanced treatments. As healthcare systems cater to the needs of an aging population, investments in glaucoma research, development, and access to care are expected to fuel the market growth in the coming years.
Market Trends:
Recent advancements in glaucoma therapeutics have revolutionized the available treatments in the market. The use of nanoparticles with suitable structures and superior binding capabilities facilitates the bioavailability and efficacy of the drug. Sustained-release (SR) implants and other innovative drug delivery systems offer improved patient compliance and reduced side effects compared to conventional eye drops. Several companies are developing SR drug delivery systems as an alternative to topical delivery to potentially overcome barriers in treating POAG. As per the article published in PubMed Central in 2022, the only approved SR therapy for POAG by the Food and Drug Administration (FDA) is Bimatoprost SR (DurystaTM) from Allergan plc. Other SR therapies including bimatoprost ocular ring (Allergan), iDose (Glaukos Corporation), ENV515 (Envisia Therapeutics), OTX-TP (Ocular Therapeutix), OTX-TIC (Ocular Therapeutix), and latanoprost free acid SR (PolyActiva) are under investigation. SR drug delivery technology in POAG management may shift treatment paradigms and dramatically improve outcomes.
In terms of geography, the glaucoma therapeutics market is divided into North America (the US, Canada, and Mexico), Europe (Spain, the UK, Germany, France, Italy, and the Rest of Europe), Asia Pacific (South Korea, China, India, Japan, Australia, and the Rest of Asia Pacific), the Middle East & Africa (South Africa, Saudi Arabia, the UAE, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America). Asia Pacific is expected to register the fastest CAGR in the glaucoma therapeutics market during the forecast period.
Glaucoma therapeutics Market: Segmental Overview
By drug class, the market is divided into beta blockers, alpha-adrenergic agonists, prostaglandin analogs, carbonic anhydrase inhibitors, combination drugs, and others. The prostaglandin analogs segment held the largest market share in 2023. Further, the combination drugs segment is projected to register the highest CAGR from 2023 to 2031.
The market, by indication, is segmented into open angle glaucoma, angle closure glaucoma, and others. The open angle glaucoma segment held the largest glaucoma therapeutics market share in 2023 and is projected to register the highest CAGR from 2023 to 2031.
By distribution channel, the market is segmented into hospital pharmacy, retail pharmacy, and online pharmacy. The hospital pharmacy segment dominated the market in 2023.
Glaucoma Therapeutics Market: Competitive Landscape and Key Developments
Inoteck Pharmaceuticals; Merck & Co., Inc.; Novartis AG; Pfizer Inc.; Santen Pharmaceutical Co., Ltd.; Teva Pharmaceutical Industries Ltd; Takeda Pharmaceutical Company; AstraZeneca PLC; AERIE Pharmaceuticals, Inc.; Alcon; and Cipla Inc are among the leading companies profiled in the glaucoma therapeutics market report. In addition, several other players have been studied and analyzed during the study to get a holistic view of the market and its ecosystem. The market players mainly adopt product innovation strategies to meet evolving customer demands and maintain their brand image.
As per company press releases, a few recent developments by players operating in the glaucoma therapeutics market are mentioned below:
• In December 2023, the US FDA approved Glaukos Corporation’s New Drug Application (NDA) for a single administration per eye of iDose TR (travoprost intracameral implant) 75 mcg, a prostaglandin analog indicated for the reduction of intraocular pressure (IOP) in patients with ocular hypertension (OHT) or open-angle glaucoma (OAG).
• In November 2022, Alcon announced the acquisition of Aerie Pharmaceuticals, Inc. The company acquired Rocklatan (netarsudil and latanoprost ophthalmic solution) 0.02%/0.005% and Rhopressa (netarsudil ophthalmic solution) 0.02%, along with AR-15512, a Phase 3 product candidate for dry eye disease, and a pipeline of several clinical and preclinical ophthalmic pharmaceutical product candidates. Rocklatan and Rhopressa are Rho-kinase inhibitors indicated for the reduction of elevated IOP in patients with OAG or OHT. The acquisition is expected to broaden Alcon’s portfolio across glaucoma, retina, and ocular surface diseases.
• In September 2023, Thea Pharma Inc. launched IYUZEH 0.005% in the US market. IYUZEH is free of preservatives and is used to treat primary open-angle glaucoma (POAG) and ocular hypertension (OHT) in the US. In randomized and controlled clinical trials, IYUZEH has reduced IOP in patients with primary open-angle glaucoma (POAG) or OHT with a baseline IOP of 19–24 mmHg by 3–8 mmHg.
• In February 2021, Santen Pharmaceutical Co., Ltd and Ube Industries, Ltd. announced that Santen Pharmaceutical Korea Co., Ltd. launched the EYBELIS Ophthalmic Solution 0.002% following Korea’s national health insurance listing. Santen and Ube Industries co-developed EYBELIS to treat glaucoma and OHT. Omidenepag Isopropyl, the active pharmaceutical ingredient in EYBELIS, licensed out from Ube Industries to Santen, is a selective EP2 receptor agonist and is an ocular hypotensive agent with a new mechanism of action.