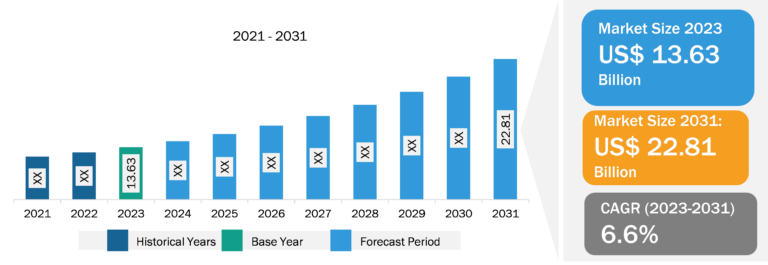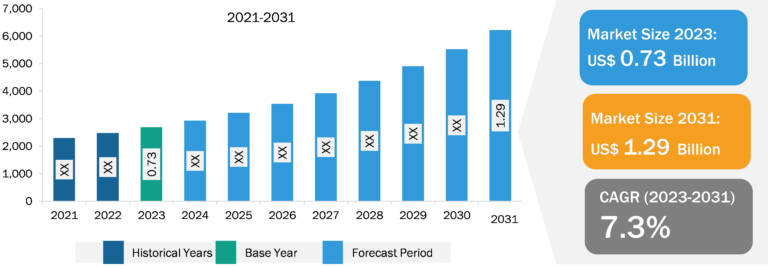
Hospital Acquired Infection Treatment Market
Asia Pacific is the fastest-growing market for hospital acquired infection treatment. North America accounted for the largest share of the global hospital acquired infection treatment market in 2022. The US held the largest hospital acquired infection treatment market share in the region in 2022 and is estimated to continue its dominance from 2022 to 2030. The US has a high prevalence of HAI, which propels the hospital acquired infection treatment market growth. As per the CDC, on any given day, about 1 in 31 hospital patients has at least one HAI. Similarly, according to the Office of Disease Prevention and Health Promotion, HAIs and other infections can lead to sepsis, and it causes an ∼1.7 million illness cases and 270,000 deaths per year in the US. Thus, the high prevalence of HAI among people in the US fuels the hospital acquired infection treatment market growth.
Furthermore, according to the US Centers for Medicare & Medicaid Services, the national healthcare expenditures in the US increased by 2.7% in 2021, reaching US$ 4.3 trillion or US$ 12,914 per person. Also, health spending accounted for 18.3% of the nation’s Gross Domestic Product. Furthermore, according to the US Department of Health & Human Services, national health spending is expected to grow at a 5.4% annual rate from 2019 to 2028, and it is expected to reach US$ 6.2 trillion by 2028. The rising health expenditure is estimated to increase various fundings in the research related to developing HAI drugs, fueling the hospital acquired infection treatment market growth.
Growing Focus on Patient Safety and Quality Care Drives Hospital Acquired Infection Treatment Market Growth
The heightened emphasis on patient safety within healthcare facilities drives the necessity for robust infection treatment strategies, infection surveillance systems, and antimicrobial stewardship programs aimed at minimizing the impact of HAIs. Ongoing investments in healthcare infrastructure, including establishing state-of-the-art hospitals and expanding medical facilities, necessitate advanced infection control and treatment measures to uphold patient safety and minimize HAI risk.
Stringent regulations, guidelines, and quality standards imposed by healthcare regulatory authorities worldwide drive the adoption of standardized infection control measures, antibiotic stewardship programs, and evidence-based treatment protocols to mitigate the burden of HAIs.
Hospital Acquired Infection Treatment Market: Segmental Overview
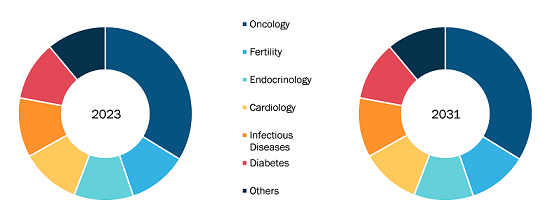
By drug class, the hospital-acquired infection treatment market is segmented into antibacterial, antiviral, antifungal, and others. The antibacterial drugs segment held the largest market share in 2022 and is anticipated to register the highest CAGR during 2022–2030. The hospital acquired infection treatment market, by infection type, is segmented into urinary tract infections, ventilator-associated pneumonia, surgical site infections, bloodstream infections, and other hospital infections. The urinary tract infections segment held the largest market share in 2022. However, the ventilator-associated pneumonia segment is anticipated to register the highest CAGR from 2022 to 2030. The hospital acquired infection treatment market, by distribution channel, is segmented into hospital pharmacies, retail pharmacies, e-commerce, and others. In 2022, the hospital pharmacies segment held the largest market share, and the same segment is anticipated to register the highest CAGR during 2022–2030. The hospital acquired infection treatment market, based on geography, is segmented into North America (the US, Canada, and Mexico), Europe (Germany, France, Italy, the UK, Russia, and the Rest of Europe), Asia Pacific (Australia, China, Japan, India, South Korea, and the Rest of Asia Pacific), Middle East & Africa (South Africa, Saudi Arabia, the UAE, and the Rest of Middle East & Africa), and South & Central America (Brazil, Argentina, and the Rest of South & Central America).
Hospital Acquired Infection Treatment Market: Competitive Landscape and Key Developments
Merck & Co Inc, Pfizer Inc, AbbVie Inc, Paratek Pharmaceuticals Inc, Eugia US LLC, Abbott Laboratories, Cumberland Pharmaceuticals Inc, Innoviva Specialty Therapeutics Inc, Cipla Ltd, and Eli Lilly and Company are a few key companies operating in the hospital acquired infection treatment market. These companies adopt product innovation strategies to meet evolving customer demands, which allows them to maintain their brand name in the hospital acquired infection treatment market.
A few of the recent developments in the global hospital acquired infection treatment market are mentioned below:
- In May 2023, Innoviva Specialty Therapeutics announced that the US Food and Drug Administration (FDA) had approved XACDURO (sulbactam for injection; durlobactam for injection), co-packaged for intravenous use in patients aged 18 years and above for the treatment of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex (Acinetobacter). Innoviva Specialty Therapeutics stated that it is focused on delivering innovative therapies in critical care and infectious diseases.
- In May 2023, Researchers of the Indian Institute of Science Education and Research (IISER), Pune, and Central Drug Research Institute (CSIR-CDRI), Lucknow, announced that they had discovered a potential new antibiotic against Acinetobacter baumannii, which frequently causes HAIs.
- In September 2020, Shionogi & Co Ltd announced that the FDA had approved a supplemental New Drug Application (sNDA) for FETROJA (cefiderocol) for the treatment of patients aged 18 years and above with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible Gram-negative microorganisms such as Enterobacter cloacae complex, Klebsiella pneumoniae, Acinetobacter baumannii complex, Escherichia coli, Pseudomonas aeruginosa, and Serratia marcescens.
- In June 2020, Merck announced that the US Food and Drug Administration (FDA) had approved a supplemental New Drug Application (sNDA) for RECARBRIO (imipenem, cilastatin, and relebactam) for the treatment of patients aged 18 years and above suffering from hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP), caused by susceptible Gram-negative microorganisms such as Acinetobacter calcoaceticus-baumannii complex, Klebsiella oxytoca, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Haemophilus influenzae, Klebsiella aerogenes, Pseudomonas aeruginosa, and Serratia marcescens.