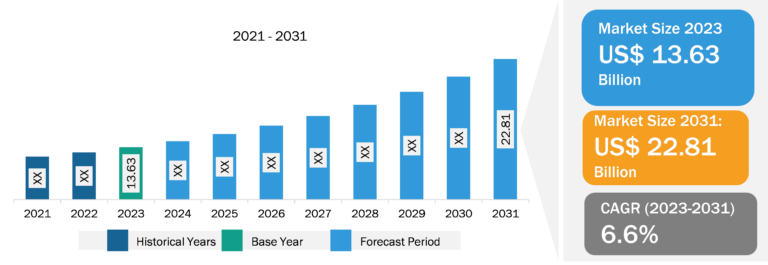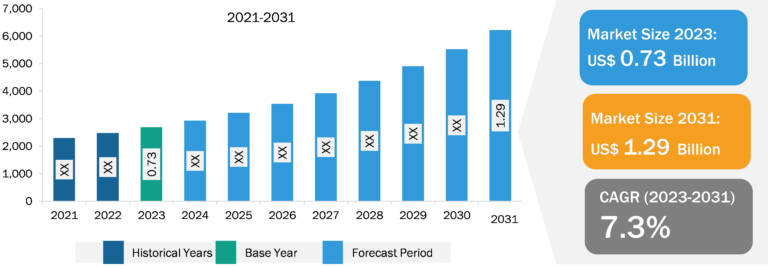
Malaria Treatment Market
Extending Pipeline of New Drugs and Vaccines
Antimicrobial resistance can put the public at risk of many diseases, such as malaria. To overcome this challenge, the R&D of new medicines is considered the best strategy; as a result, the antimalarial pipeline is becoming increasingly robust. Public-private partnerships are accelerating research activities related to the treatment of malaria. For instance, Medicines for Malaria Venture (MMV), a nonprofit organization, works with numerous pharmaceutical companies to reduce the burden of malaria by developing novel treatment options.
A few antimalarial that are under clinical development are mentioned in the table below:
Malaria Drugs/Vaccine/Compounds Under Clinical Development
| Drug | Developer | Clinical Trial Stage |
| Artefenomel | Sanofi | Phase IIb |
| KAE609 | Novartis AG | Phase IIb |
| KAF156 | Novartis AG | Phase IIb |
| DSM265 | Takeda Pharmaceutical Company | Phase IIa |
| SANARIA PfSPZ-GA1 | Sanaria | Phase II |
| SANARIA PfSPZ Vaccine–Radiation Attenuated PfSPZ Vaccine | Sanaria | Phase II |
| SANARIA PfSPZ-CVac | Sanaria | Phase II |
| MSP3-CRM-Vac4All | Vac4All | Phase I |
| Liver-Stage Antigen-3 (LSA3) | Vac4All | Phase IIa |
Source: Company Website, The Insight Partners Analysis
Therefore, the growing number of antimalarial drugs/vaccines that are under clinical development are expected to boost the market growth in the coming years.
The Middle East & Africa holds the largest share of the malaria treatment market, with Nigeria, Ghana, Uganda, Angola, and Cameroon being the major contributors to the market growth in this region. However, Asia Pacific is anticipated to register the highest CAGR during the forecast period. The growth of the malaria treatment market is driven by the collaborations between the companies, growing incidences of cancer, and increasing clinical trials. Moreover, India reported an 83.34% reduction in malaria morbidity and a 92% decrease in associated mortality from 2000 to 2019. The Centers for Disease Control and Prevention (CDC) claims that pregnant women are three times more susceptible to malaria infections as compared to others. Thus, rapid diagnosis and subsequent treatment plans are essential, which favor the growth of the malaria treatment market in India. There is also an escalating demand for the RDTs as they facilitate easy and rapid diagnoses. Many international organizations and the Government of India are working towards the eradication of malaria. The introduction of the Parasight Malarial Platform for hospitals and pathology labs in India by companies such as Becton, Dickinson and Company and SightDX for the rapid diagnosis of malaria also favors the market growth. The increasing number of latent malaria cases in the country and the demand for advanced diagnostics tests are driving the growth of the malaria treatment market.
Malaria Treatment Market: Segmental Overview
Based on treatment, the global malaria treatment market is segmented into generic drugs, originators, vaccines, and others. The vaccines segment held the largest share of the market in 2022; moreover, the same segment is anticipated to record the highest CAGR of 28.9% during the forecast period. Vaccines provide active acquired immunity against particular infectious diseases. The market for malaria vaccines is growing at a significant growth rate owing to increasing research and development activities and an increasing pipeline of vaccine candidates. Increasing research and development activities are offering lucrative opportunities for the segment growth. For instance, in March 2020, Novavax, Inc. and Serum Institute of India entered into a commercial license agreement; this will allow SII to use Novavax’s proprietary Matrix-M vaccine adjuvant with SII’s malaria vaccine candidate. Matrix-M is a key component in the malaria vaccine candidate and is currently in a Phase 2b clinical trial sponsored by the Jenner Institute, UK.
Malaria Treatment Market: Competitive Landscape and Key Developments
Cipla Ltd, Sun Pharmaceutical Industries Ltd, Sanofi SA, GSK Plc, Novartis AG, Pfizer Inc, Emmaus Life Sciences Inc, AdvaCare Pharma USA LLC, VLP Therapeutics LLC, Lupin Ltd, and Teva Pharmaceutical Industries Ltd. are among the leading companies operating in the global malaria treatment market. These players are focusing on expanding, diversifying their market presence, and acquiring a novel customer base, thereby tapping prevailing business opportunities.
- In July 2022, the Gavi Vaccination Project, funded by the Gates Foundation, introduced the Mosquirix vaccine developed by GlaxoSmithKline (GSK) for the first time in three African countries: Kenya, Ghana, and Malawi. This vaccine was reportedly the first malaria vaccine. This was a critical first step in introducing malaria vaccines in countries with moderate to high transmission rates of malaria caused by P. falciparum.
- In May 2021, Novartis delivered 1 billion courses of antimalarial treatment, including 430 million pediatric treatments, largely at no profit since 1999. More than 90% of this artemisinin-based combination therapy (ACT) was supplied without profit to malaria-endemic countries across the globe.
- In January 2021, GSK, PATH, and Bharat Biotech (BBIL) declared that a product transfer agreement was signed for the malaria vaccine RTS,S/AS01E. The agreement included transferring the manufacturing of the RTS,S antigen portion of the vaccine and granting a license for all rights related to the malaria vaccine to BBIL. GSK retained the production of the vaccine adjuvant (AS01E) and supplied it to BBIL.
- In May 2020, ADVANZ PHARMA Corp. Limited acquired Correvio Pharma Corp, a specialty pharmaceutical company. Under the acquisition, ADVANZ PHARMA achieved an immediate and direct commercial expansion in France, Germany, Italy, Spain, and the Benelux region.