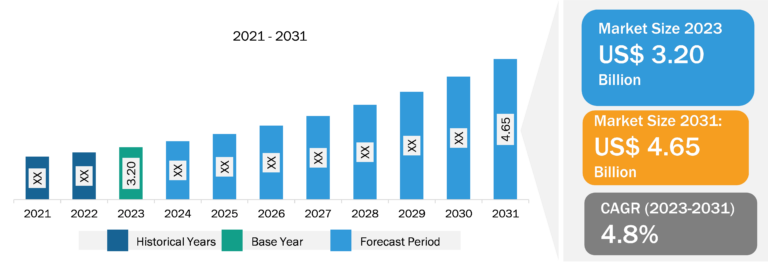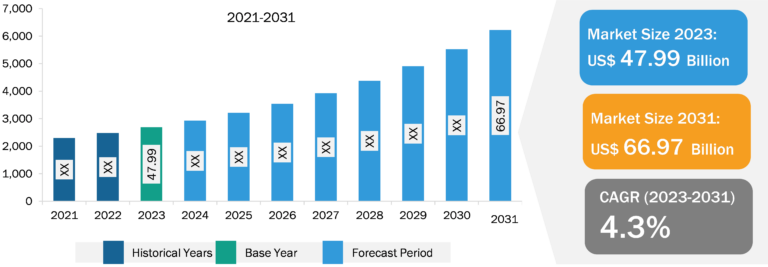
Fill Finish Manufacturing Market
The term “fill/finish” describes the final steps of the manufacturing process, which are filling and finishing. Filling in the pharmaceutical industry means putting medication into a container and closing it, whereas finishing refers to sterilizing and standardizing medical supplies and containers.
Low Business Costs in Emerging Markets to Create Opportunity for Fill Finish Manufacturing Market Growth During Forecast Period
Many Asian countries are emerging as attractive outsourcing locations for various biopharmaceutical manufacturers across the globe. Low manufacturing and operating costs in China, India, and other countries in Asia are the key factors boosting contract manufacturing. Moreover, recent developments in the biopharmaceutical industry in China and India indicate significant potential for the market. The growing pipelines of biologics and biosimilars are further opening new avenues for the contract manufacturers in APAC. Contract manufacturing mostly benefits early-stage drug innovators owing to lower operational costs. To meet the growing demand, many CMOs are expanding their manufacturing capabilities. For instance, in January 2020, STA Pharmaceutical Co., Ltd—a WuXi AppTec company—opened a new large-scale oligonucleotide active pharmaceutical ingredient (API) manufacturing facility in China.
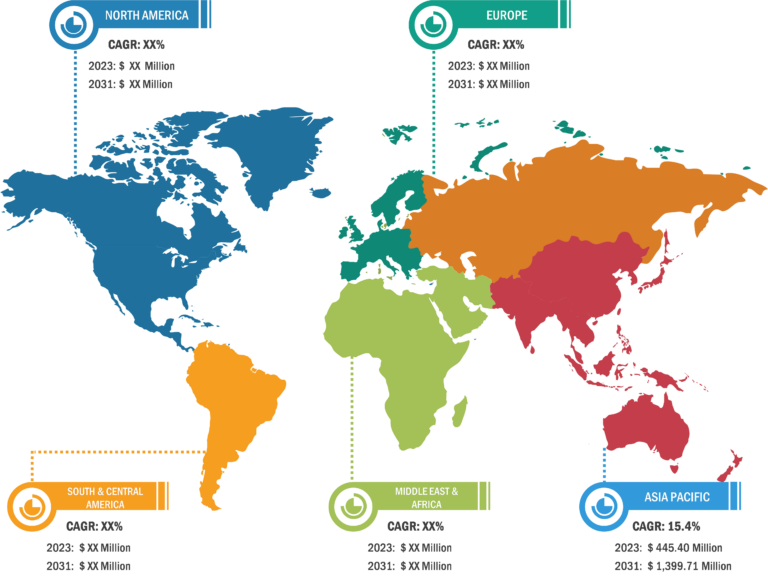
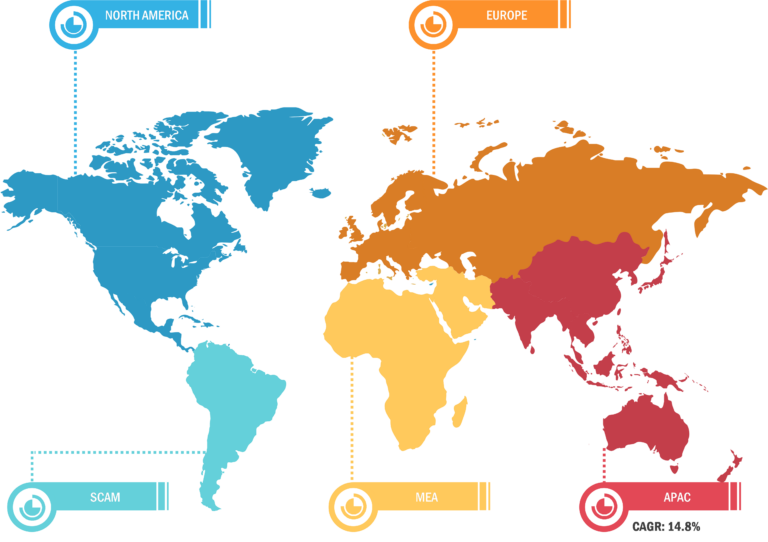
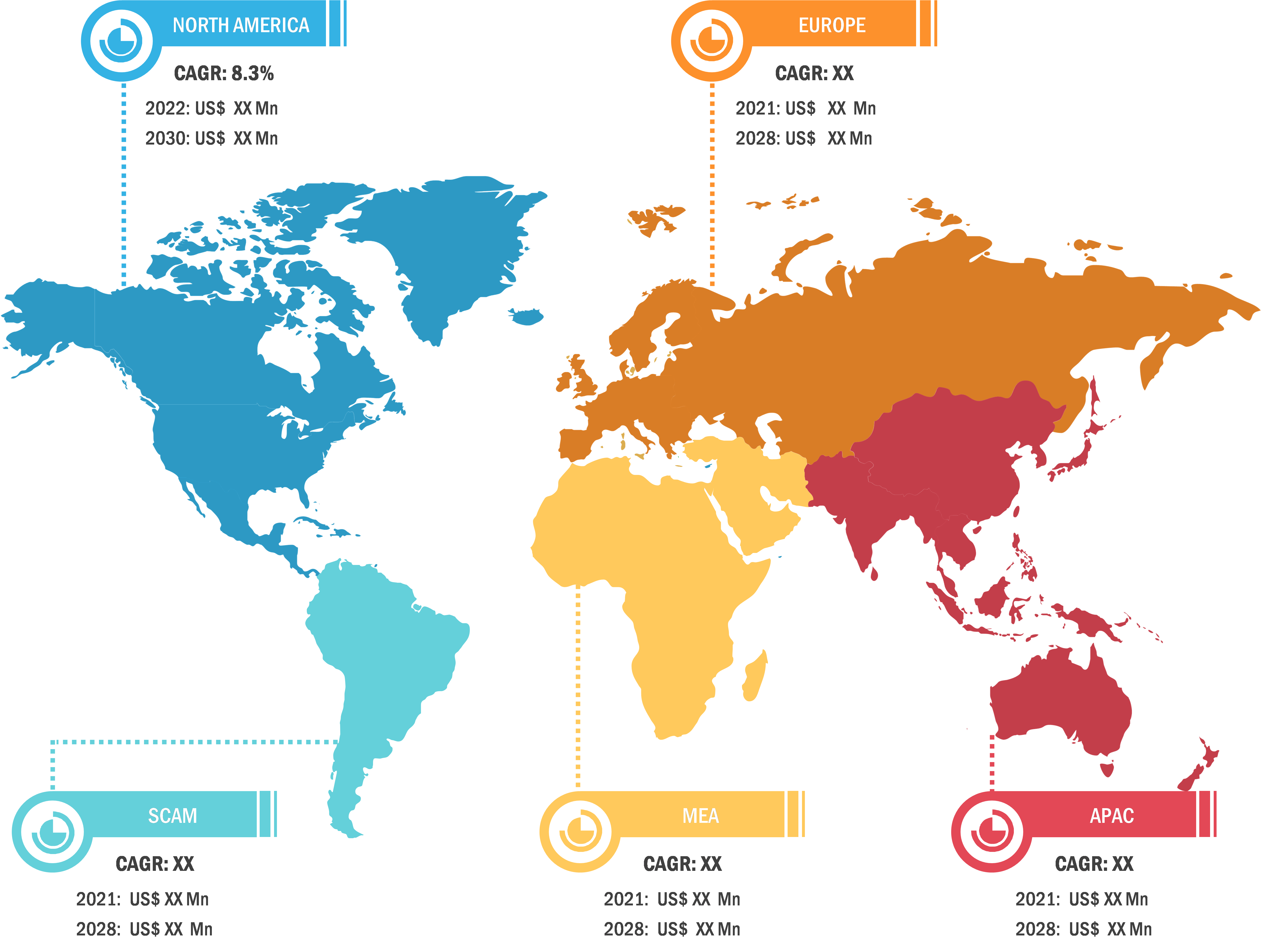
North America held the largest share of the global fill finish manufacturing market in 2022. The fill finish manufacturing market in the US is anticipated to be the largest and fastest-growing market in the world. Several factors, such as extensive research and development activities and advanced manufacturing of innovative biopharmaceutical and pharmaceutical products, lead to the growth of the market. The other leading factor for market growth is sustained diversity and large-scale supply of biopharmaceutical and pharmaceutical products across the globe. The biopharmaceutical industry is among the largest revenue-generating sector in the country. In 2023, it generated more than 1.3 million jobs in the US. Thus, the growth in the biopharmaceutical industry has substantially assisted in maintaining the country’s total economic balance. It generates ~US$ 550 million in revenue annually. Therefore, it is expected that the rise in research and product development will increase the demand for lyophilization services in the US.
In addition, various service providers in the country offer a range of fill/finish solutions. The majority of service providers are private organizations. For instance, Baxter Healthcare Corporation offers fill finish service through its business unit, BioPharma Solutions. It collaborates with pharmaceutical companies to assist them in commercializing their products by providing scientific expertise, sterile contract manufacturing solutions, parenteral delivery systems, and customized support services. Moreover, BioPharma Solutions offers biologics contract manufacturing using innovative filling line technologies that are particularly designed to protect the biologic’s integrity and optimize the yield of costly API. Similarly, Emergent BioSolutions, Ajinomoto Bio-Pharma Services, Cobra Biologics, Cook Pharmica, and Dalton Pharma Services are a few other service providers in the country. Further, on November 16, 2020, Vibalogics—a Germany-based contract development and manufacturing organization (CDMO)—announced that it had begun its three-year, US$ 150 million investment into a late-phase clinical and commercial virotherapy manufacturing facility near Boston, MA; thus, establishing its presence in the US.
On November 24, 2020, Baxter International Inc.—a global leader in sterile medication production and delivery—announced an investment of US$ 50 million for the expansion of its sterile fill/finish manufacturing facilities in Bloomington, Ind. Baxter’s BioPharma Solutions business operates these facilities; it is a premier contract manufacturing company that specializes in parenteral (injectable) pharmaceuticals. A combination of Baxter and client investment is funding the expansion. The pre-planned expansion of existing facility infrastructure includes the construction of a new 25,000-sq-ft warehouse, a high-speed automated syringe fill line proficiently filling approximately 600 units per minute, a new filling line for flexible plastic containers, and a new high-speed automated visual inspection line.
Fill Finish Manufacturing Market: Competitive Landscape and Key Developments
IMA Industria Macchine Automatiche SpA, Nipro Medical Europe NV, Maquinaria Industrial Dara SL, Groninger and Co GmbH, SGD SA, Optima Packaging Group Gmbh, NNE AS, Stevanato Group SpA
Syntegon Technology GmbH, West Pharmaceutical Services Inc, Gerresheimer AG, Schott AG, and Becton Dickinson and Co are a few key companies operating in the fill finish manufacturing market. Market players adopt product innovation strategies to meet evolving customer demands, thereby maintaining their brand names in the fill finish manufacturing market.
A few recent developments in the global fill finish manufacturing market are mentioned below:
- In February 2023, Gerresheimer and Corning announced a joint venture to meet the rising demand for Velocity Vials. The partnership aims to integrate Gerresheimer’s extensive glass converting expertise with the Velocity Vial technology platform by Corning, helping set a new standard for the industry. Pharmaceutical companies are expected to benefit from enhanced filling line performance with high throughput and yield, thereby reducing their total cost of ownership.
- In April 2022, West Pharmaceutical Services Inc. entered an exclusive supply and technology agreement with Corning Inc. This collaboration involves a multimillion-dollar investment to further expand the Valor Glass technology by Corning and enable advanced injectable drug packaging and delivery systems for the pharmaceutical industry in order to improve patient safety and expand access to life-saving treatments. This partnership and investment enable the development of solutions that enhance patient safety, boost quality and reliability in highly regulated markets, and ensure greater capacity for life-saving drugs.