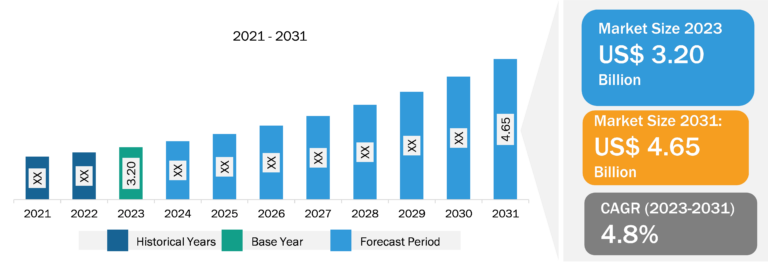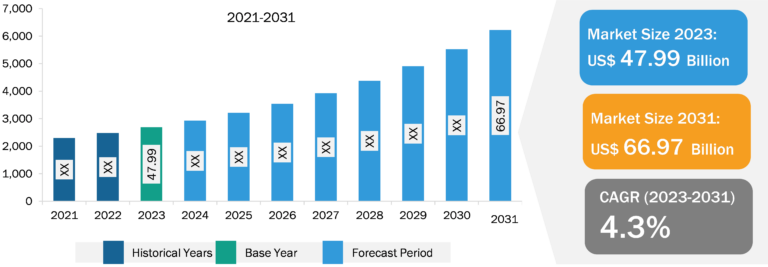
Microbiome Therapeutics Market
The potential of gut microbiota in maintaining human health and inflow of funds to ameliorate microbiome discovery pipeline bolster the microbiome therapeutics market size. However, the high cost of development and production hinders the microbiome therapeutics market growth.
Increasing R&D to Gain Better Understanding of Human Microbiome is creating Opportunity for Microbiome Therapeutics Market Growth
The growing recognition of the microbiome’s importance in human health and disease has encouraged rigorous research and development efforts to further understand the human microbiome and develop microbiome therapeutics. It has helped develop potential therapeutics and identify hindrances. Advancements in technology and research tools have also contributed to growing R&D. Next-generation sequencing and metagenomic analysis have allowed for a more comprehensive and detailed study of the microbiome. These tools enable researchers to identify specific microbial species and their functions, as well as track changes in the microbiome over time. Regulatory agencies, such as the FDA, are also supporting R&D efforts in the field of microbiome therapeutics. The FDA has established guidelines for FMT and has developed interest in advancing the field through regulatory pathways to ensure safety and efficacy. This support from regulatory agencies provides a framework for companies to develop and test microbiome-based therapies, giving investors confidence in the potential success of these treatments. Overall, the increased interest and funding in microbiome therapeutics are driving more research and development efforts. This will lead to a better understanding of the human microbiome and the development of targeted therapies that can modulate the microbiome to improve human health.
Technological advancements have facilitated the development of targeted interventions. By understanding the specific microbial species that are associated with certain diseases or conditions, researchers can develop therapies that specifically target those microbes. This personalized approach has the potential to be more effective and have fewer side effects compared to traditional broad-spectrum antibiotics. In addition to advancements in sequencing technology, other technological innovations have also contributed to the development of microbiome therapeutics. Furthermore, there has been progress in the development of synthetic biology tools that allow for the engineering of microbial communities with desired functions. This opens up new possibilities for designing microbiome-based therapies that can be tailored to individual patients.
North America held the largest share of the global microbiome therapeutics market in 2022 owing to the growing number of lung cancer patients, increasing habit of smoking among the youth, government support for lung cancer screening, and the presence of major market players engaged in new and existing product developments. The US held the largest share of the microbiome therapeutics market in North America in 2022, and it is anticipated to register the highest CAGR during 2022–2030. The increasing understanding of the importance of the human microbiome in maintaining health and preventing diseases has led researchers in the US to explore microbiome-based interventions as a promising approach to treat various conditions. Additionally, the dominance of this country in the microbiome therapeutics market is mainly attributed to its well-developed research infrastructure that favors the development of innovative treatments Further, the rising prevalence of chronic diseases such as obesity and inflammatory bowel disease has created a demand for innovative treatments, spurring the development of microbiome therapeutics.
Technological advancements in gene sequencing and bioinformatics enable the study and alteration of the microbiome, facilitating the development of personalized microbiome-based interventions. The trend toward personalized medicine aligns well with tailormade microbiome therapeutics. Collaborations between academia, industry, and government organizations are also fueling research and development efforts, accelerating the translation of scientific discoveries into commercial products. Regulatory support and investment in the field further contribute to market growth, as regulatory bodies recognize the potential of microbiome therapeutics and provide guidance for their development and approval.
An emphasis on research and innovation has yielded a significant understanding of the impact of gut microbiota on health, providing a solid foundation for microbiome-based therapeutics. A supportive regulatory environment for biotechnology and pharmaceuticals in Canada has encouraged companies to invest in microbiome-focused research and development. Additionally, increased venture capital investments and government funding have infused capital into this research. In January 2020, the Government of Canada, along with its partners, invested US$ 18 million in crucial microbiome research.
The rising awareness among Canadians about the importance of gut health and microbiome in overall well-being has spurred consumer demand for microbiome-based products. Moreover, the widespread implementation of initiatives such as the Canadian Microbiome Initiative (CMI) makes working with partners and stakeholders easier in Canada. This makes it possible to concentrate research efforts on the development of microbiome therapies. Collaborations and partnerships between academic institutions, biotechnology firms, and healthcare organizations have accelerated research and development efforts. Moreover, Canadian companies are exploring international markets, expanding the reach of microbiome therapeutics. All these factors combined indicate a promising trajectory for microbiome therapeutics in Canada.
Microbiome Therapeutics Market: Competitive Landscape and Key Developments
Enterome, Finch Therapeutics Group Inc, Caelus Health, Ferring Pharmaceuticals, Pendulum Therapeutics Inc., AOBiome, Seres Therapeutics, Vedanta Biosciences, COST-BRY Pty Ltd (BiomeBank), and YSOPIA Bioscience are a few key companies operating in the microbiome therapeutics market. Market players adopt product innovation strategies to meet evolving customer demands, thereby maintaining their brand names in the microbiome therapeutics market.
A few recent developments in the global microbiome therapeutics market are mentioned below:
- In November 2023, the company obtained its first worldwide regulatory approval for a donor-derived microbiome medicinal product in November 2022 from the Therapeutic Goods Administration (TGA).
- In February 2023, Ferring Pharmaceuticals announced that RBX2660, also known as Rebyota, is a fecal microbiota product that can be used for treatment in the US. Ferring has provided adults with a much-needed new therapeutic option for recurrent C diff infection with the introduction of Rebyota. The introduction of this revolutionary live biotherapeutic based on microbiota offers an innovative approach to treating people with recurrent C diff (CDI) infection.