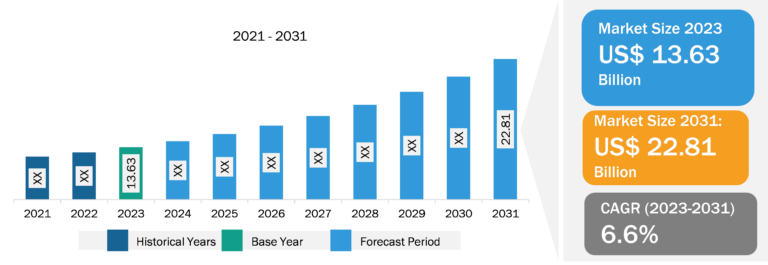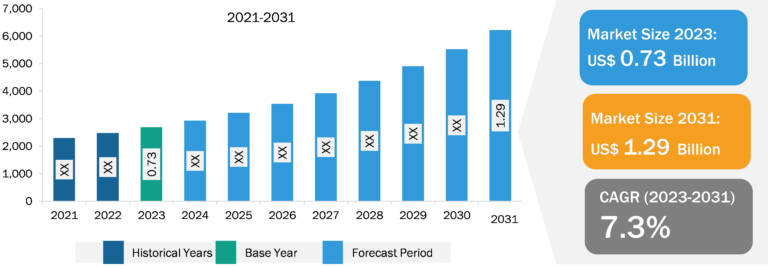
Drug Discovery Services Market
Increasing Prevalence of Chronic Diseases is Driving The Drug Discovery Services Market Growth
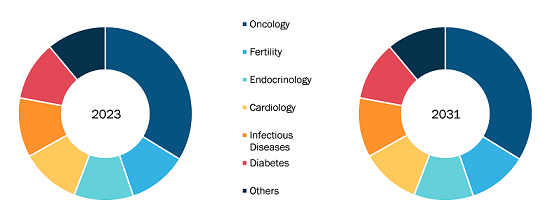
According to data published by the Centers for Disease Control and Prevalence (CDC) in 2022, there would be ~37.3 million people with diabetes, which is 11.3% of the US population. As the prevalence of chronic and complex diseases continues to rise, there is an urge for innovative treatments that can address unmet medical needs and improve patient outcomes. Furthermore, the number of people aged 60 and above has increased due to an upsurge in life expectancy. According to the United Nations Database on World Population Ageing 2020, the number of elderly individuals worldwide is expected to increase from 727 million in 2020 to 1.5 billion by 2050. The geriatric population is more susceptible to chronic illnesses, which propels the demand for chronic disease management. Drug discovery services play an important role in the development of new drugs and medical technologies. Companies providing these services help pharmaceutical companies and research institutions identify and validate potential drug targets, conduct preclinical studies, and optimize drug candidates for clinical trials, which are all essential for bringing new and effective treatments to market for addressing the growing burden of chronic diseases.
The growing focus on the fields of personalized and precision medicine further emphasizes the demand for discovery services. The personalized approach of developing tailored treatments for individual patients based on the genetic makeup of individuals, along with other factors, requires extensive research and discovery to identify the most effective treatments for the unique needs of each patient. Thus, the drug discovery services market would continue to grow as the healthcare sector strives to address the rising burden of chronic diseases such as cancer, cardiovascular diseases, respiratory diseases, and diabetes, and improve patient outcomes.
The process of discovering and developing new drugs is expensive and often requires substantial investments in research, development, and clinical trials. As evaluated in the Genetic Engineering & Biotechnology study, the average cost of creating a new medication, determined considering the world’s top 20 biopharmaceuticals, soared by 15% (US$ 298 million) to reach almost US$ 2.3 billion in 2022. Additionally, the high failure rate of drug candidates in the later stages of development further adds to the overall cost. As a result, pharmaceutical and biotechnology companies are increasingly seeking cost-effective drug discovery services, compelling service providers to offer competitive pricing while maintaining high-quality standards. The high cost of drug molecules also impacts the affordability of new therapies for patients, especially in the case of rare diseases or niche therapeutic areas, which is a major area of challenge for both drug developers and healthcare systems amid the process of providing access to innovative treatments.
The majority of global research and development funds for pharmaceuticals and biologics are devoted to North America. The growth of the drug discovery services market in North America is attributed to a rise in investments made by drug development companies, large grants made available by the US government, the strong presence of major drug development companies, a well-developed healthcare infrastructure, and an increase in the incidence of chronic diseases. The American Cancer Society’s “Cancer Facts & Figures 2022” estimated ~1,918,030 new cancer cases and 609,360 cancer-related deaths in the US by the end of 2022. Given the high rate of cancer in this area, research activities to innovate cancer drugs are likely to flourish in the US in the coming years. CytoReason and Pfizer signed a multi-year cooperation in September 2022. According to this deal, Pfizer can use CytoReason’s artificial intelligence technology for the development of medicines. The use of AI technology in drug discovery by major companies in the US is anticipated to propel the expansion of the drug discovery services market.
Drug Discovery Services Market: Competitive Landscape and Key Developments
Abbott Laboratories Inc., Agilent Technologies Ubiquigent, Albany Molecular Research Inc., Advinus Therapeutics, AstraZeneca PLC, Bayer AG, Aurigene, Charles River Laboratories International, ChemBridge Corporation, and Covance are a few of the key companies operating in the drug discovery services market. Market players adopt product innovation strategies to meet evolving customer demands, thereby maintaining their brand names in the drug discovery services market.
A few recent developments by drug discovery services market players are mentioned below:
- In January 2023, the US Food and Drug Administration (FDA) accepted Genentech’s Biologics License Application (BLA) and granted Priority Review for this treatment. It approved the use of Glofitamab, an investigational CD20xCD3 T-cell engaging bispecific antibody, for the treatment of adult patients with relapsed or refractory (R/R) large B-cell lymphoma (LBCL), following two or more lines of systemic therapies. As an aggressive (fast-growing) form of non-Hodgkin’s lymphoma (NHL), LBCL is among the most common adult blood cancers in the US. The FDA had planned to decide on the approval of this breakthrough cancer immunotherapy by July 1, 2023. If authorized, glofitamab would be the first commercially available, fixed-duration, CD20xCD3 T-cell engaging bispecific antibody for treating patients with aggressive lymphomas, who have had several chemotherapy sessions.
- In February 2020, PharmaResources—a contract research organization (CRO) located in Shanghai, China—and Eurofins Discovery, a drug discovery services provider, disclosed a combined drug discovery platform that could expedite the development of small molecule medications for clinical use.