CAR-T Cell Therapy Market
Increase in Number of Product Approvals Drives Market Growth
CAR-T cell therapy is a novel and cutting-edge method of cancer immunotherapy. It is a treatment in which a patient’s T cells are genetically modified in laboratories with chimeric antigen receptors (CAR) to target cancer cells. In both adults and children, CAR-T cell therapy shows promising results for the treatment of various forms of leukemia and other blood cancers. As a result, five CAR-T cell therapies have received approval from the US Food and Drug Administration (FDA), which is in charge of evaluating novel therapies and approving them for sale.
Targeted treatment, faster and more efficient recovery, and reduced side effects are among the advantages of cell therapy. Globally, cell therapies are widely adopted owing to the availability of FDA-approved products. For instance, FUCASO (Equecabtagene Autoleucel)—the first fully human BCMA-directed CAR-T cell therapy for adult patients with relapsed or refractory conditions co-developed and co-commercialized by IASO Biotechnology and Innovent Biologics, Inc.—has been approved by China’s National Medical Products Administration (NMPA). Innovent Biologics, Inc. is a world-class biopharmaceutical company that develops, manufactures, and markets high-quality medicines for the treatment of cancer, autoimmune diseases, metabolic disorders, eye disorders, and other major diseases. IASO Biotechnology is a clinical-stage biopharmaceutical company involved in discovering, developing, and manufacturing innovative cell therapies and antibody products.
In June 2022, Bristol Myers Squibb received FDA approval for Breyanzi (lisocabtagene maraleucel), a CD19-directed CAR-T cell therapy for the treatment of adult patients with large B-cell lymphoma (LBCL).
Therefore, the increasing number of approvals for T cell therapies is fueling the market growth.
Market Opportunity
Increase in Research and Development Activities by Manufacturers
The CAR-T cell therapies are progressing at a notable pace with multiple drug approvals, a rich pipeline, and many ongoing clinical trials. Pipelines of CAR-T cell therapies are in various stages of clinical development; major pharmaceutical companies are working to advance the pipeline and future enhance the potential of CAR-T cell therapy. Thus, key players are investing substantially in research and development. In September 2023, a collaborative research agreement was signed by the National Cancer Center and Asahi Kasei Corp. to develop cellular immunotherapies that make use of CAR-T cell format. Through this partnership, the Division of Cancer Immunology at the National Cancer Center Research Institute develops CAR-T cell pipelines that are used in clinical settings to treat patients with T cell malignancies and tumors that are resistant to conventional immunotherapies. JW Therapeutics drug JWCAR029 is a CAR-T cell product that targets CD19 and is intended to treat advanced lymphoma and leukemia. The molecule is currently in Phase II of development. JWCAR029 is initially being investigated for treating B-cell malignancies, focusing on relapsed and refractory DLBCL.
Thus, CAR-T cell therapies are emerging as a new trend in the T cell therapy market.
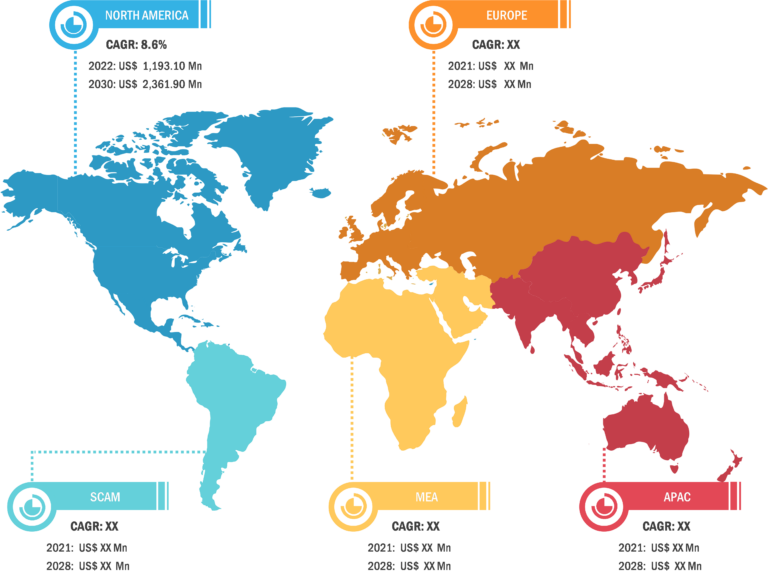
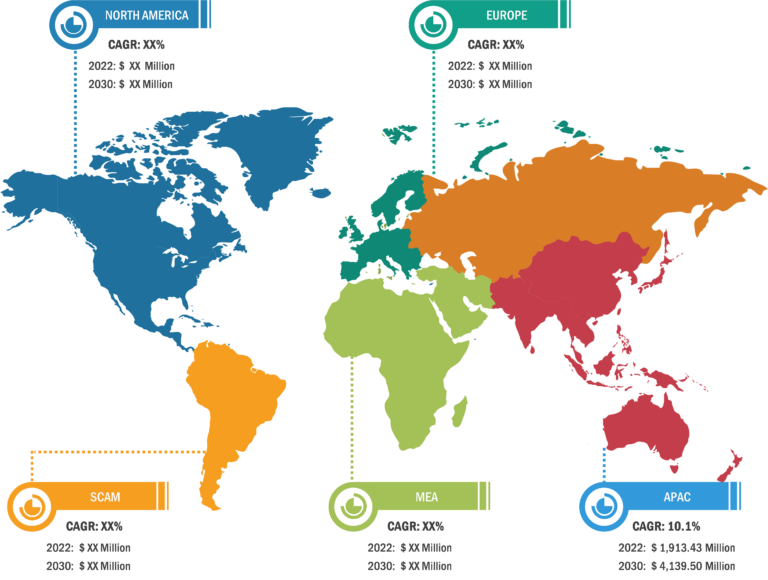
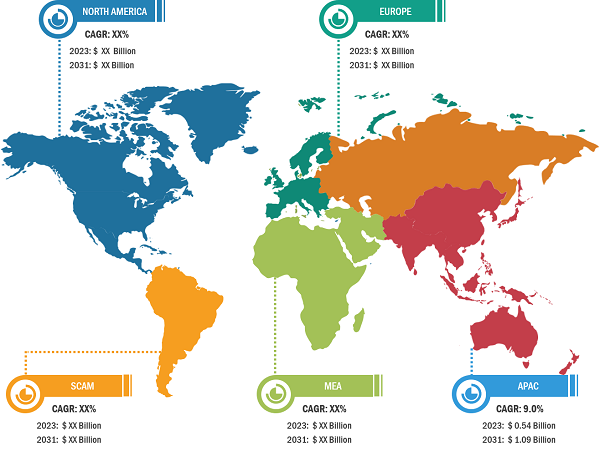
CAR-T Cell Therapy Market: Geographic Overview
Geographically, the CAR-T cell therapy market report is segmented into North America, Europe, Asia Pacific, and Rest of World. In 2022, North America accounted for the largest CAR-T cell therapy market share. Asia Pacific is expected to register the highest CAGR during 2022–2WS030. China is the fastest growing country for CAR-T cell clinical trials, which hospitals conduct[AT1] [AP2] .. China approved its first CAR-T cell therapy, relmacabtagene autoleucel of JW Therapeutics, in September 2021 for the treatment of adult patients with relapsed or refractory large B-cell lymphoma (r/r LBCL) after two or more lines of systemic therapy. Further, growing government initiatives to improve product access and increasing healthcare infrastructure are the factors likely to continue to drive the market growth. For instance, in February 2021, Novartis announced that the Therapeutic Goods Administration (TGA) approved the commercial distribution of its CAR-T therapies to eligible patients in Australia. Peter MacCallum Cancer Center in Melbourne is the first authorized commercial manufacturing site for CAR-T cell therapies in Australia.
CAR-T Cell Therapy Industry Developments and Future Opportunities:
A few strategic developments by leading players operating in the CAR-T cell therapy market are listed below:
- In December 2023, AstraZeneca entered into a definitive agreement to acquire Gracell Biotechnologies Inc., a global clinical-stage biopharmaceutical company that develops cutting-edge cell therapies for the treatment of autoimmune diseases and cancer. With GC012F, an innovative, clinical-stage FasTCAR-enabled BCMA and CD19 dual-targeting autologous CAR-T therapy, AstraZeneca’s expanding cell therapy pipeline could potentially treat multiple myeloma in addition to other hematologic malignancies and autoimmune diseases such as systemic lupus erythematosus (SLE).
- In December 2022, Kite (a Gilead company) announced the acquisition of Tmunity Therapeutics (Tmunity), a private biotech company in the clinical stage that specializes in next-generation CAR T-therapies and technologies.
CAR-T Cell Therapy Market: Competitive Landscape and Key Developments
The CAR-T cell therapy market analysis is carried out by identifying and evaluating key players in the market across different regions. Bristol-Myers Squibb Company; Novartis AG; Gilead Sciences, Inc.; Johnson & Johnson Services, Inc.; CARsgenTherapeutics Co., Ltd; Aurora Biopharma; Legend Biotech; Pfizer Inc.; bluebird bio, Inc.; Mustang Bio; Sorrento Therapeutics, Inc.; and Fate Therapeutics are the prominent players profiled in the CAR-T cell therapy market report. These companies focus on introducing new technologies, upgrading existing products, and undertaking geographic expansions to be able to address the globally increasing consumer demand.